Abstract
1. Response patterns of 116 muscle stretch receptor units isolated from the sciatic nerve of the duck have been studied, and the units classified as muscle spindles and tendon organs.
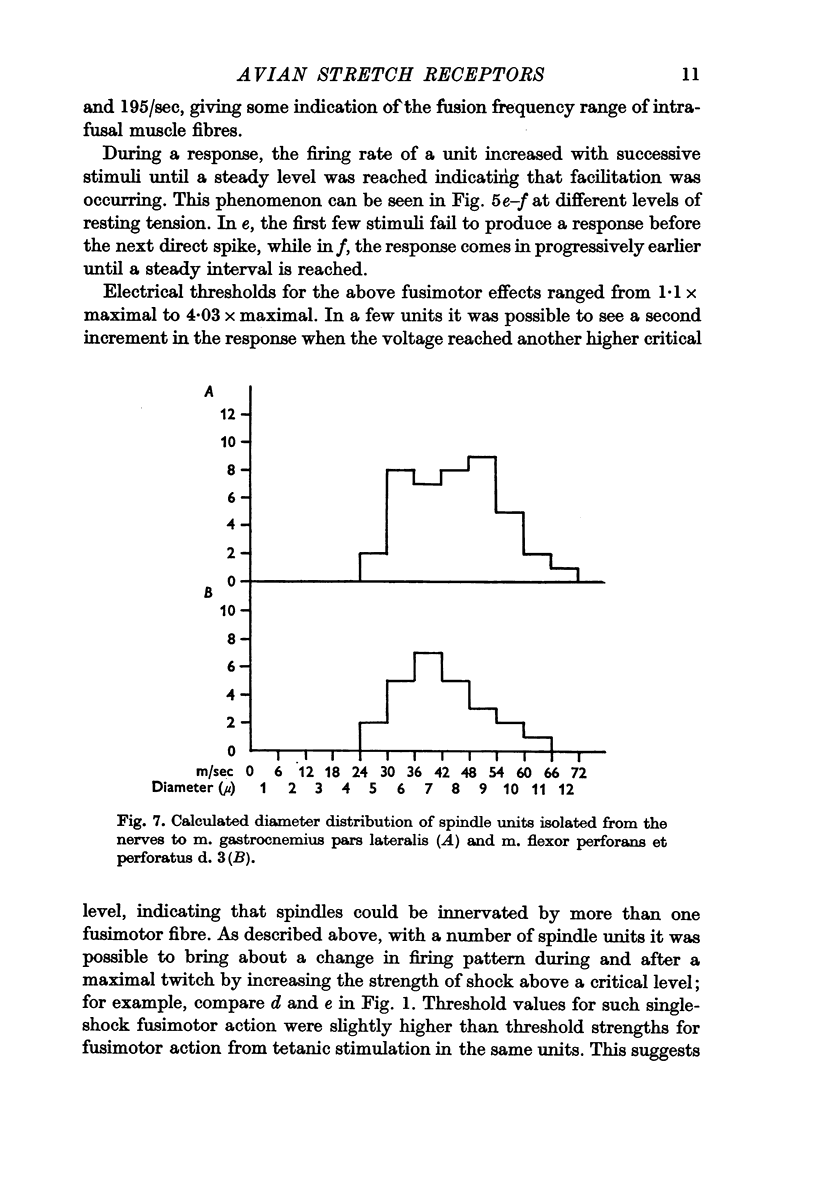
2. Units classified as spindles had low threshold tensions for maintained discharge. From conduction—velocity measurements, the calculated fibrediameter spectrum appears to be unimodal, ranging from 5 to 11-12 μm.
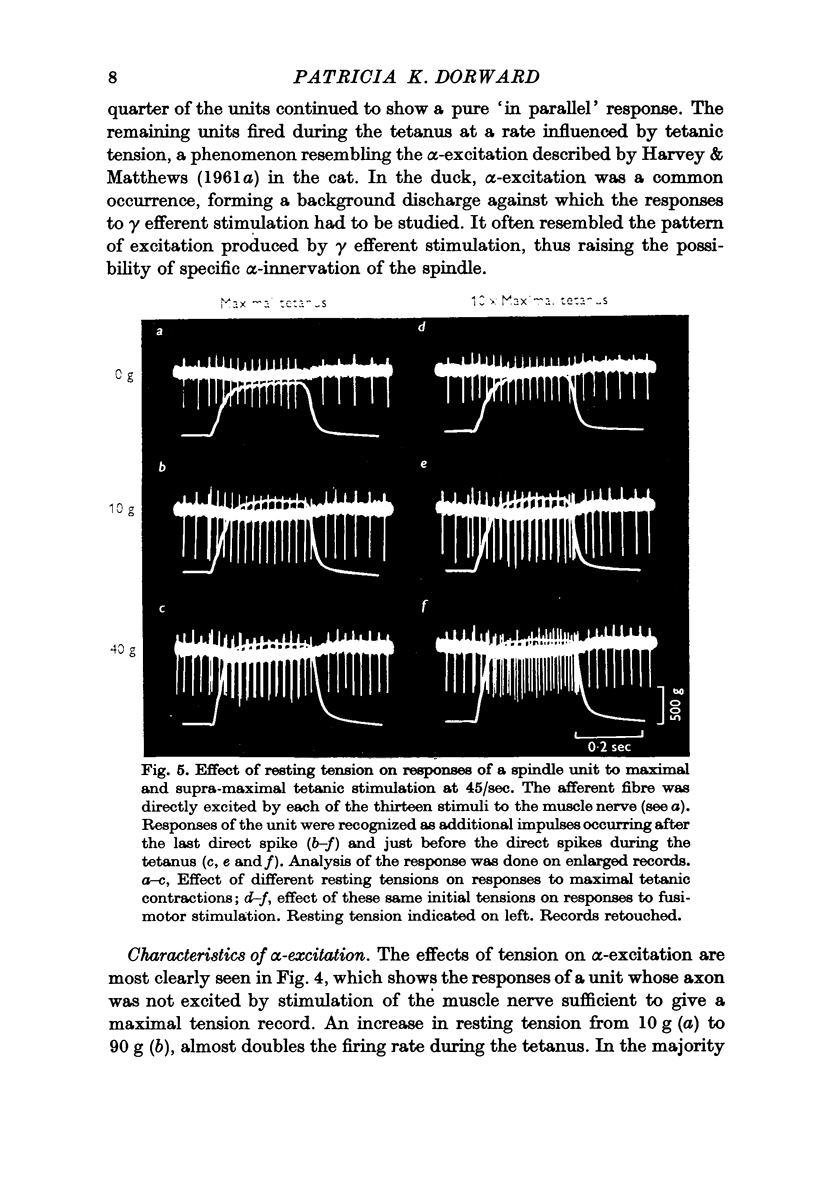
3. Spindle units showed essentially `in parallel' behaviour, though increase in initial tension often led to the appearance of `in series' responses. Although apparent `α-excitation' during maximal tetanic contractions was a common occurrence, no direct evidence of α-innervation of spindles was obtained.
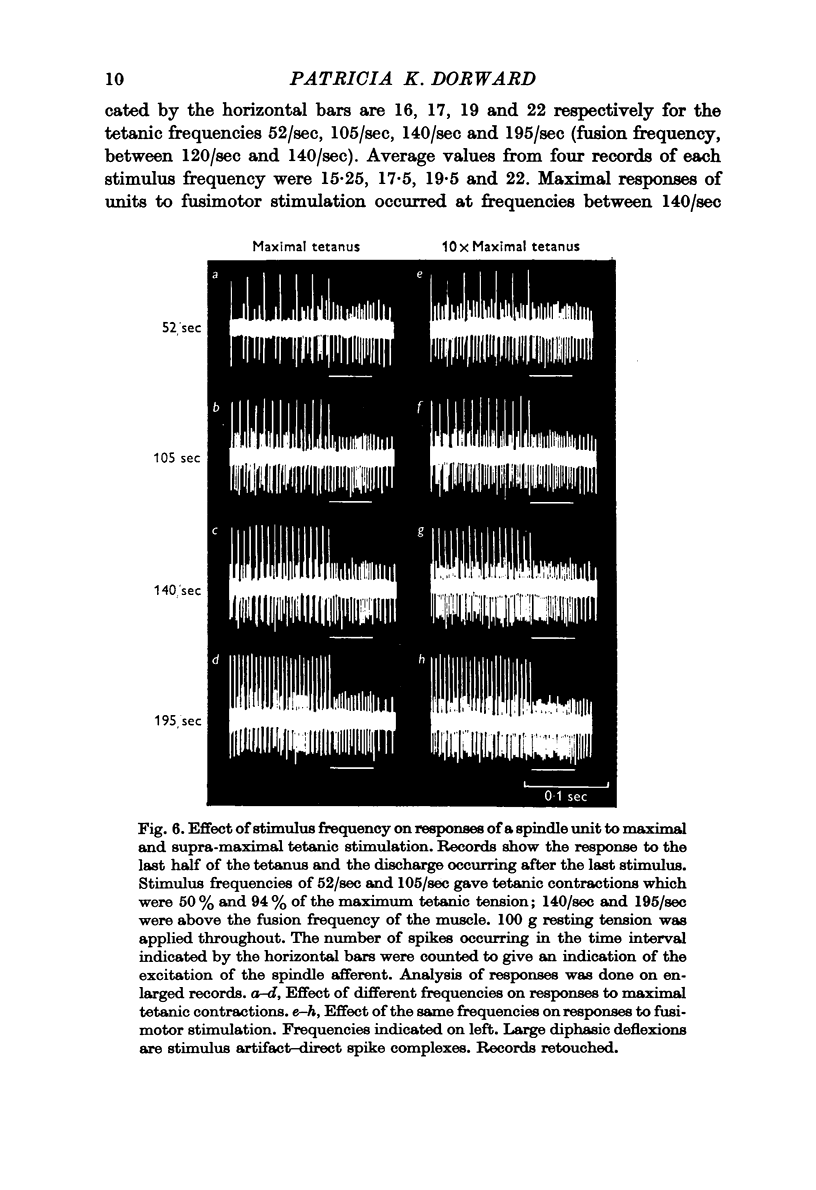
4. Evidence has been obtained for motor innervation of spindles by fibres distinct from those constituting the alpha supply to extrafusal muscle fibres. Afferent response attributable to this fusimotor innervation is influenced by initial tension and stimulus-frequency. Electrical thresholds for fusimotor responses ranged from 1·1 to 4·03 times α maximum.
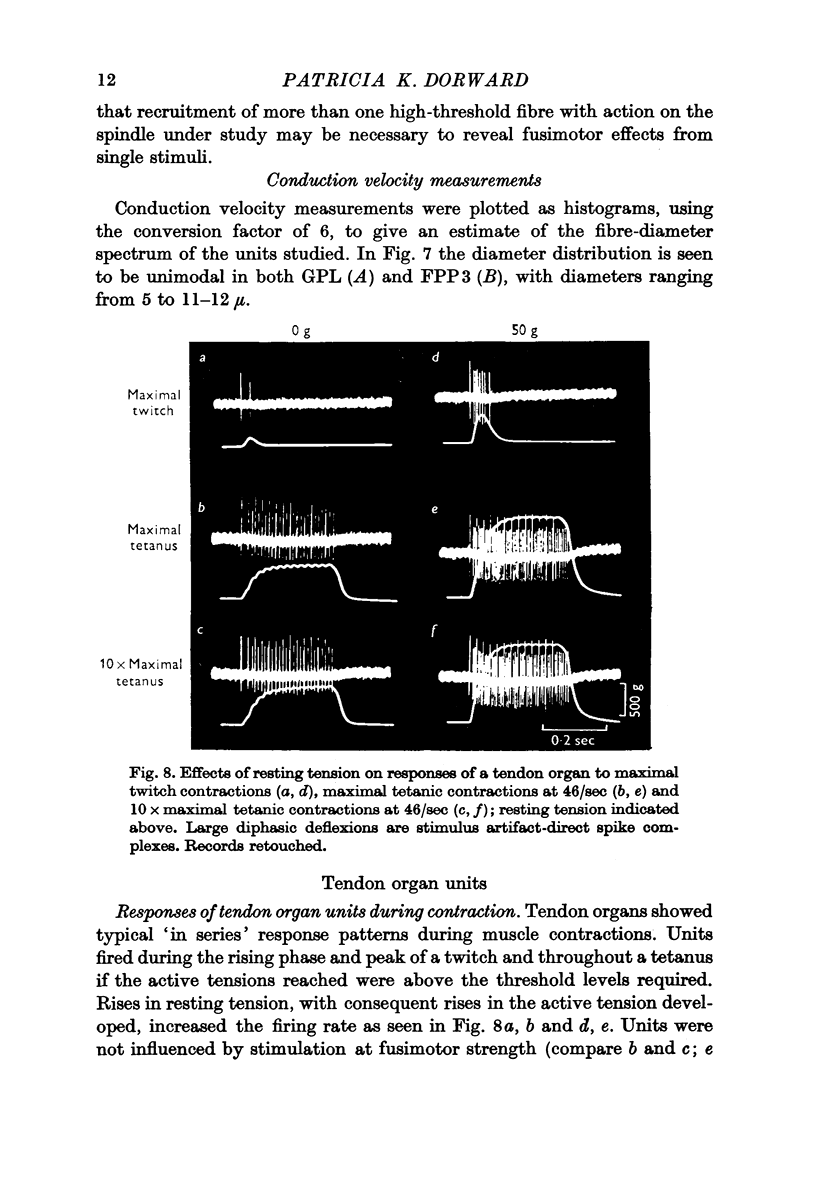
5. Tendon organ units consistently showed `in series' response patterns during muscle contractions. They were not influenced by stimulation of the high-threshold efferent nerve supply to the muscles.
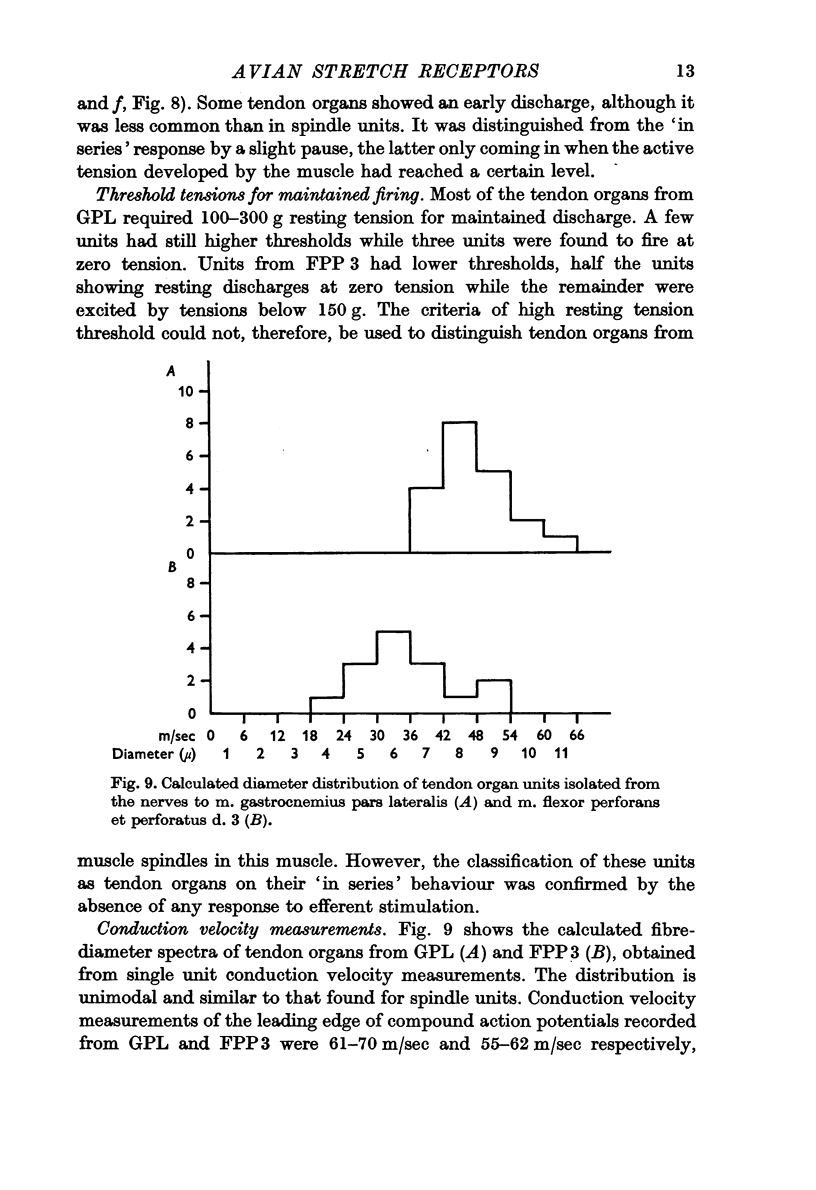
6. Threshold tensions required for maintained discharge in tendon organ units from m. gastrocnemius pars lateralis were characteristically high; however, many units from m. flexor perforans et perforatus d. 3 had unexpectedly low mechanical thresholds. The calculated fibre-diameter spectrum for tendon organ units is unimodal, ranging from 4-7 to 10-11 μm. As in mammals, they contribute to the coarse-fibre component in the muscle nerve and include the fastest fibres present.
Full text
PDF


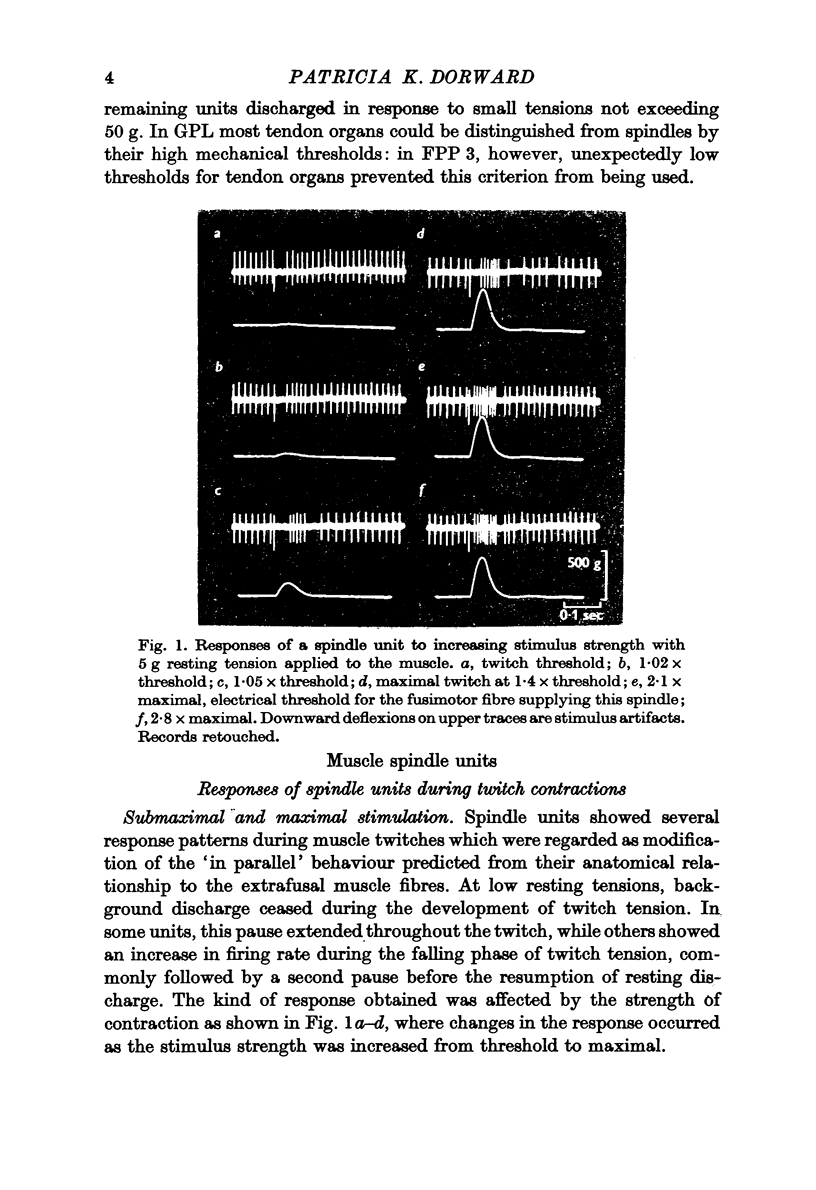
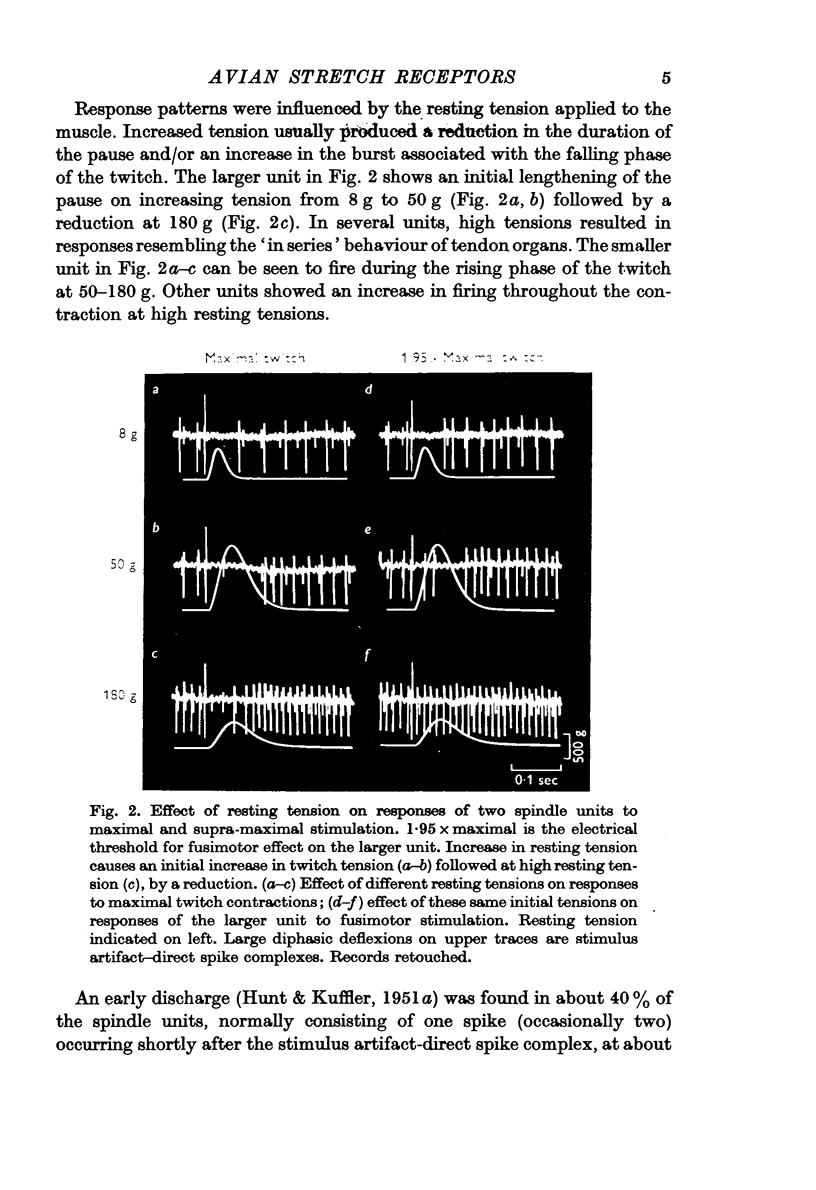
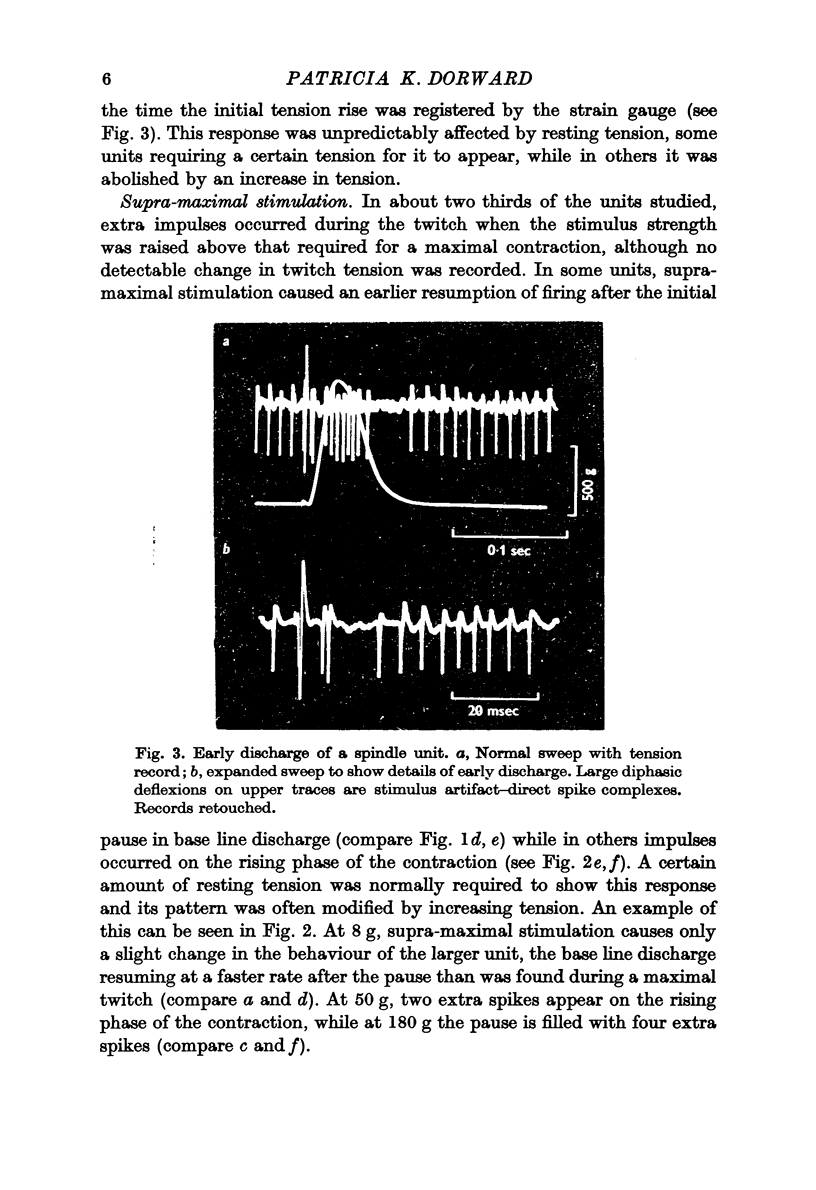
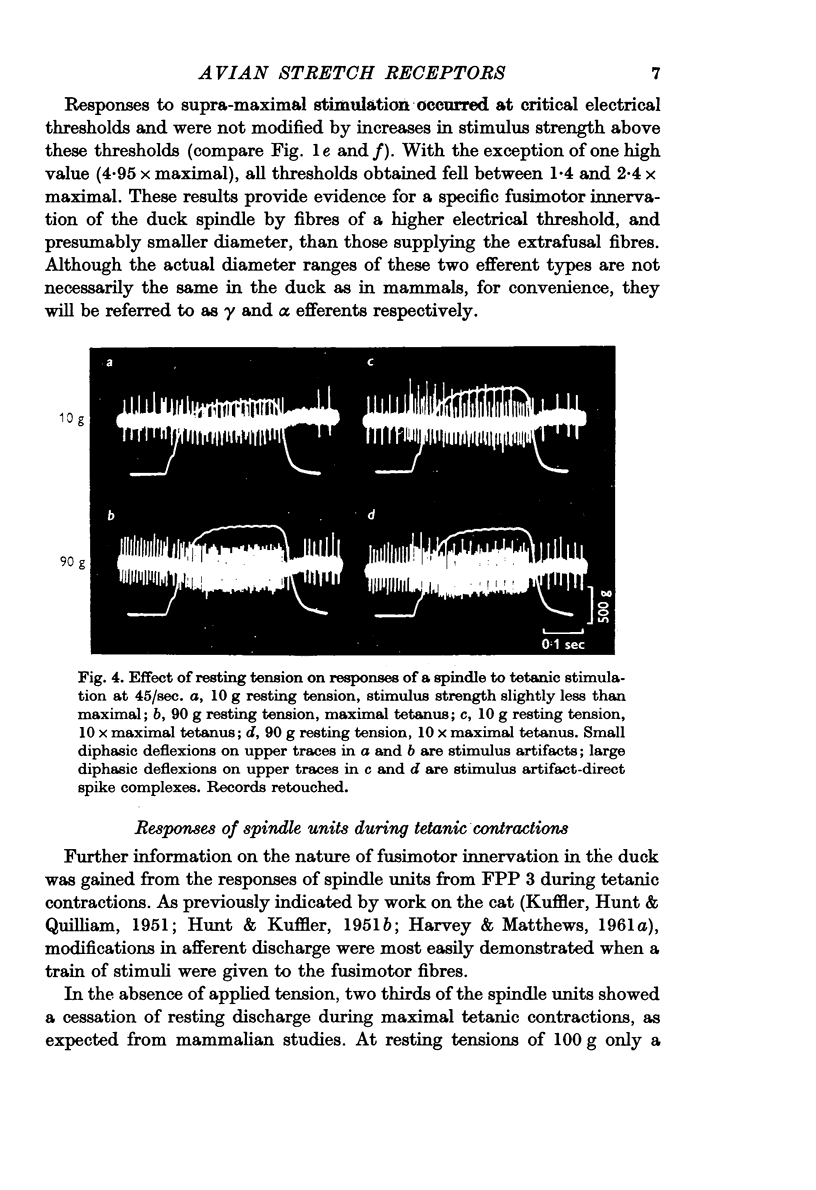





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. L'innervation motrice du muscle strié des vertébrés. Actual Neurophysiol (Paris) 1968;8:23–71. [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci. 1961 Oct;46:389–398. doi: 10.1113/expphysiol.1961.sp001558. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., MATTHEWS P. B. Some effects of stimulation of the muscle nerve on afferent endings of muscle spindles, and the classification of their responses into types A1 and A2. J Physiol. 1961 May;156:470–497. doi: 10.1113/jphysiol.1961.sp006688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., MATTHEWS P. B. The response of de-efferented muscle spindle endings in the cat's soleus to slow extension of the muscle. J Physiol. 1961 Jul;157:370–392. doi: 10.1113/jphysiol.1961.sp006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol. 1951 Apr;113(2-3):283–297. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Stretch receptor discharges during muscle contraction. J Physiol. 1951 Apr;113(2-3):298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]


