Abstract
1. The relative contribution of K and Cl to the total increase of membrane conductance produced by adrenaline on the smooth muscle of guinea-pig taenia coli has been investigated. Constant current pulses were applied with a pair of large external electrodes, the voltage change was recorded with an intracellular micro-electrode, and the change in the size of the electrotonic potential by adrenaline was measured.
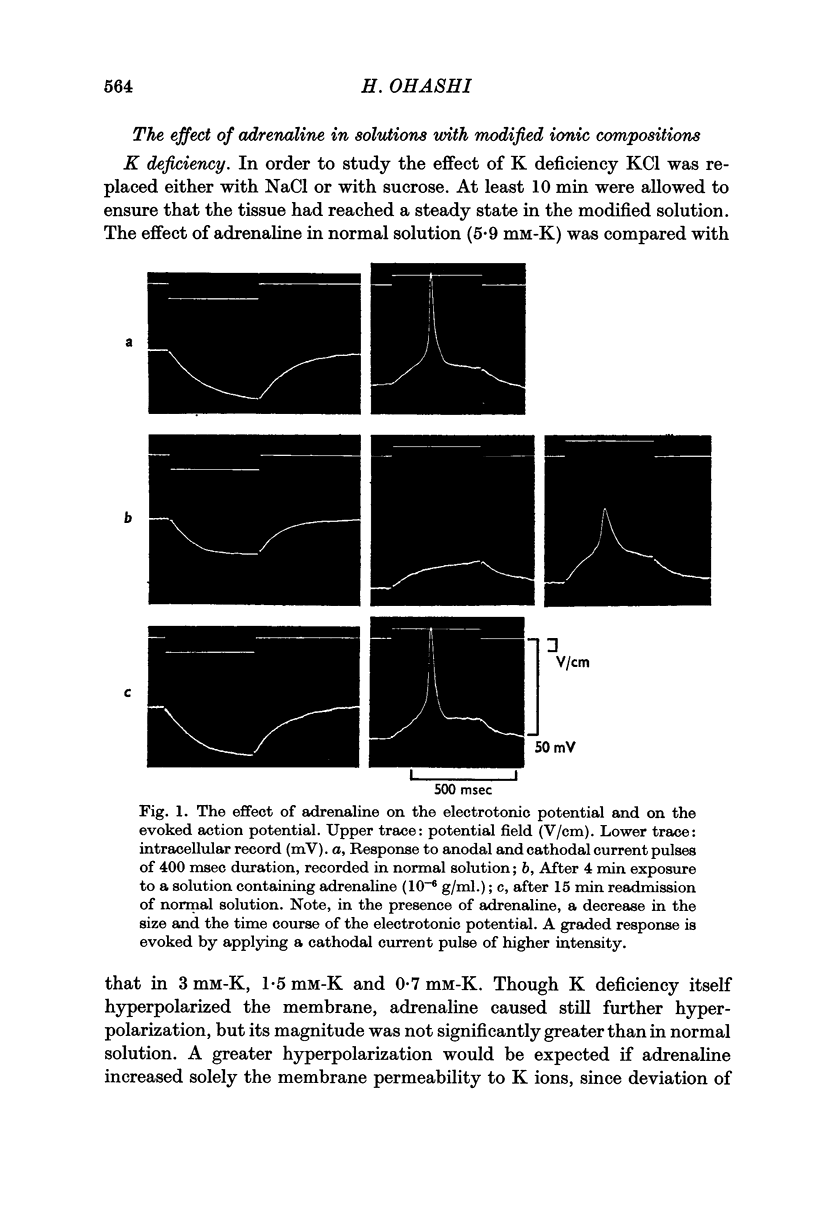
2. Adrenaline hyperpolarized the membrane by about 8 mV with a range from 5 to 15 mV and it reduced both the size and the time course of the electrotonic potential. On the average the size was reduced to 65 ± 1·8% (n = 75) of the normal size, from which a decrease of the membrane resistance to 45 ± 2·2% was calculated, or 48% allowing for the spatial decay.
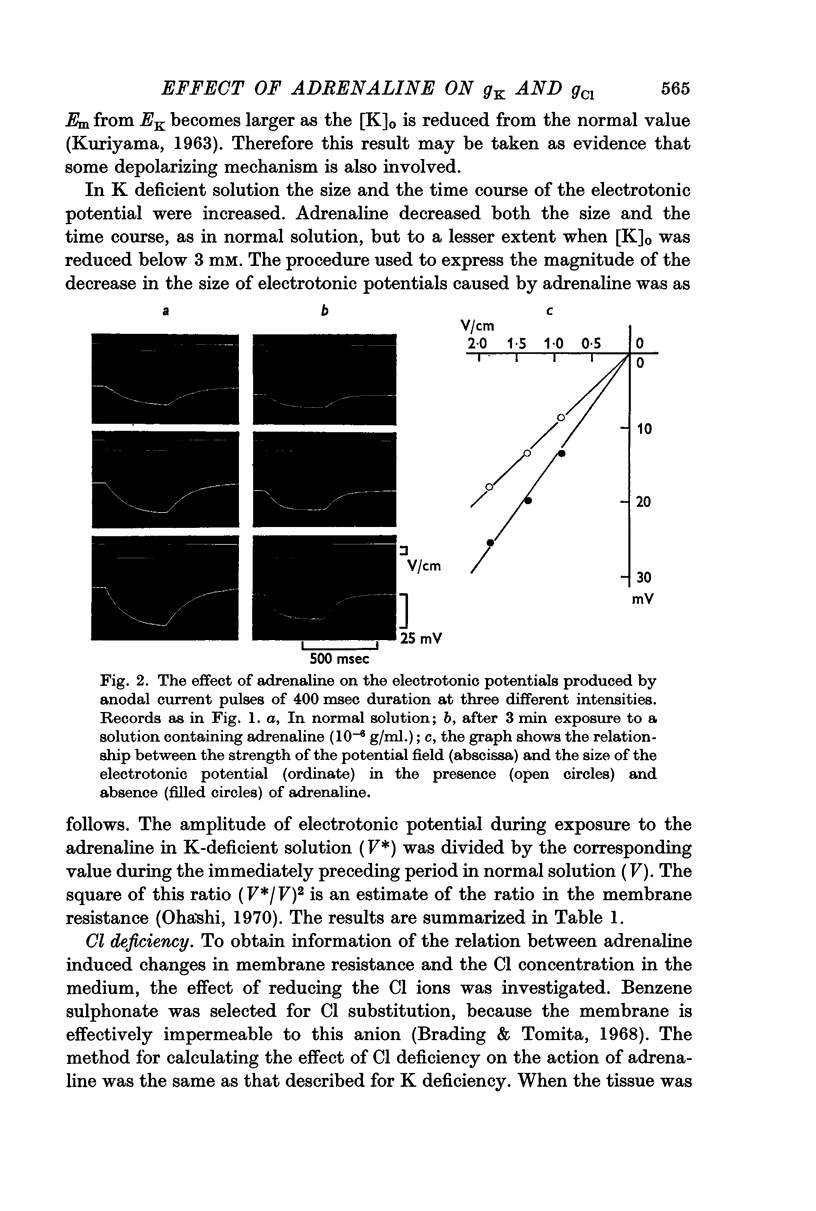
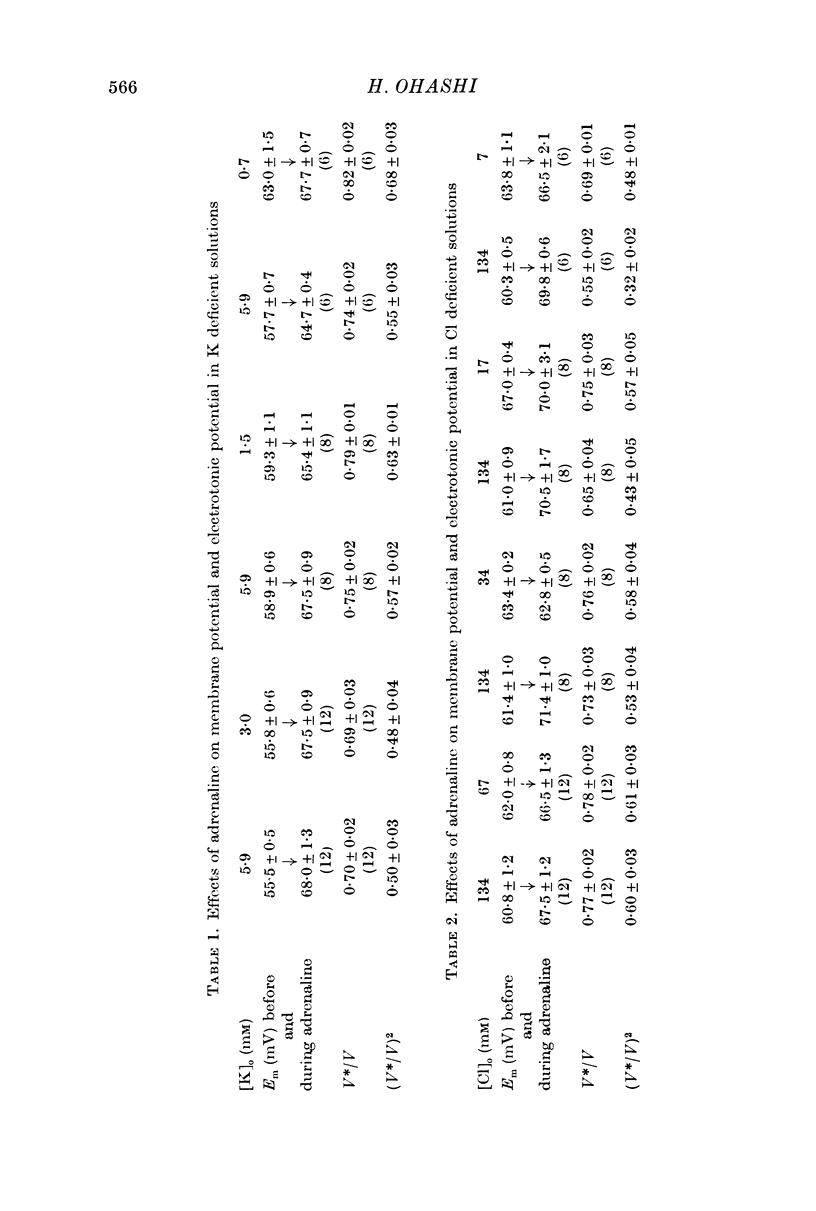
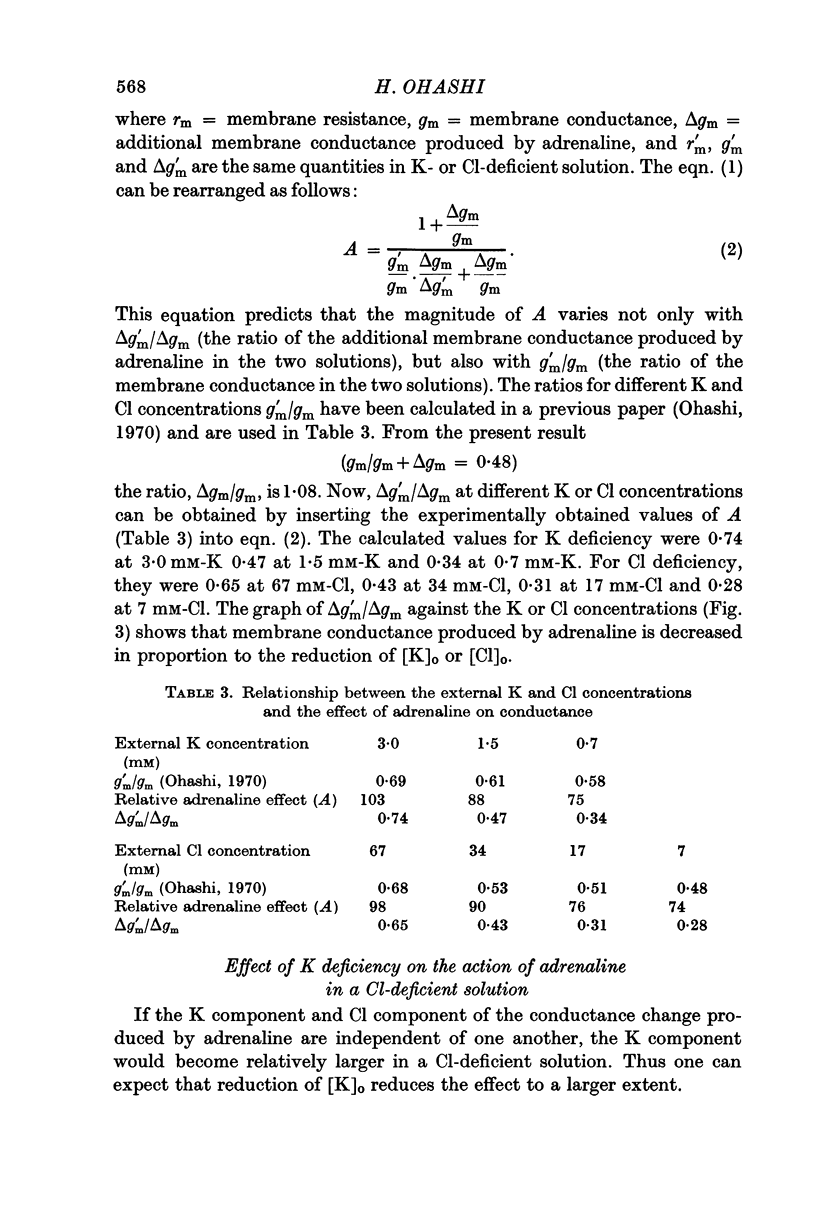
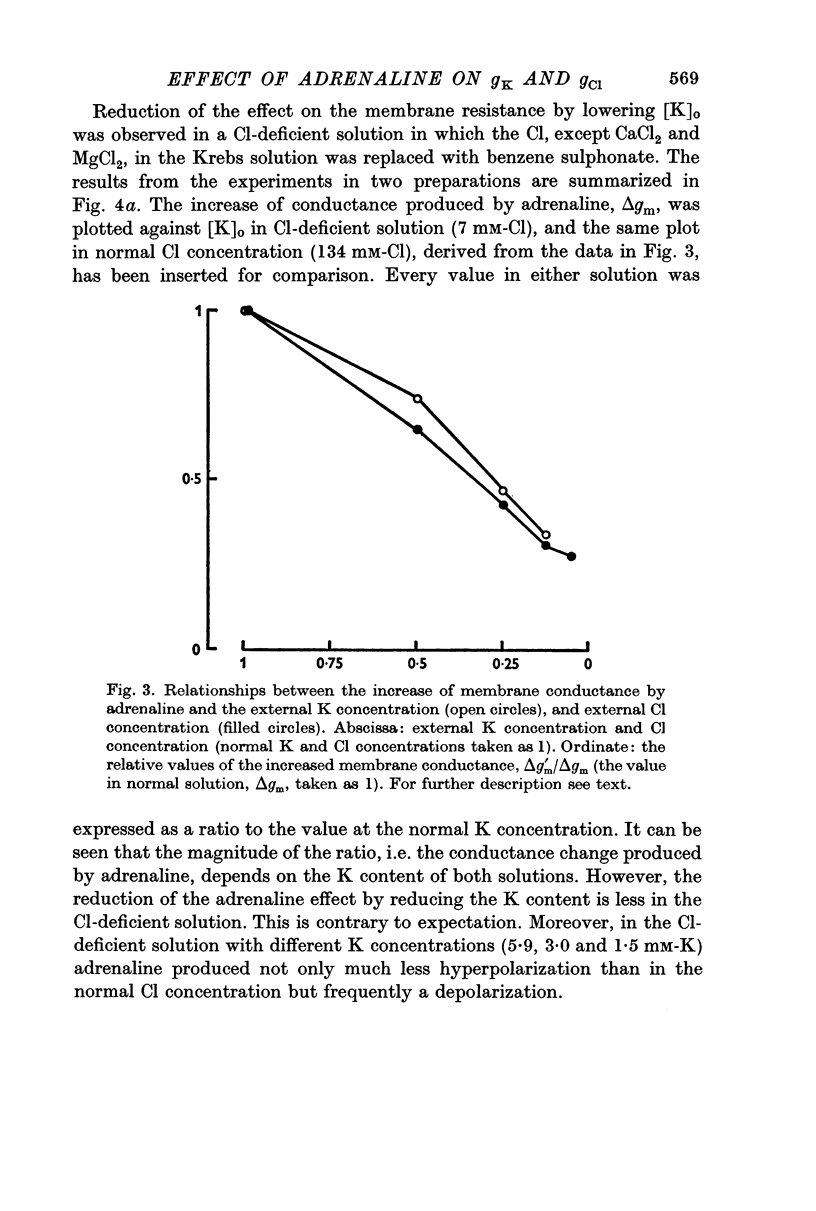
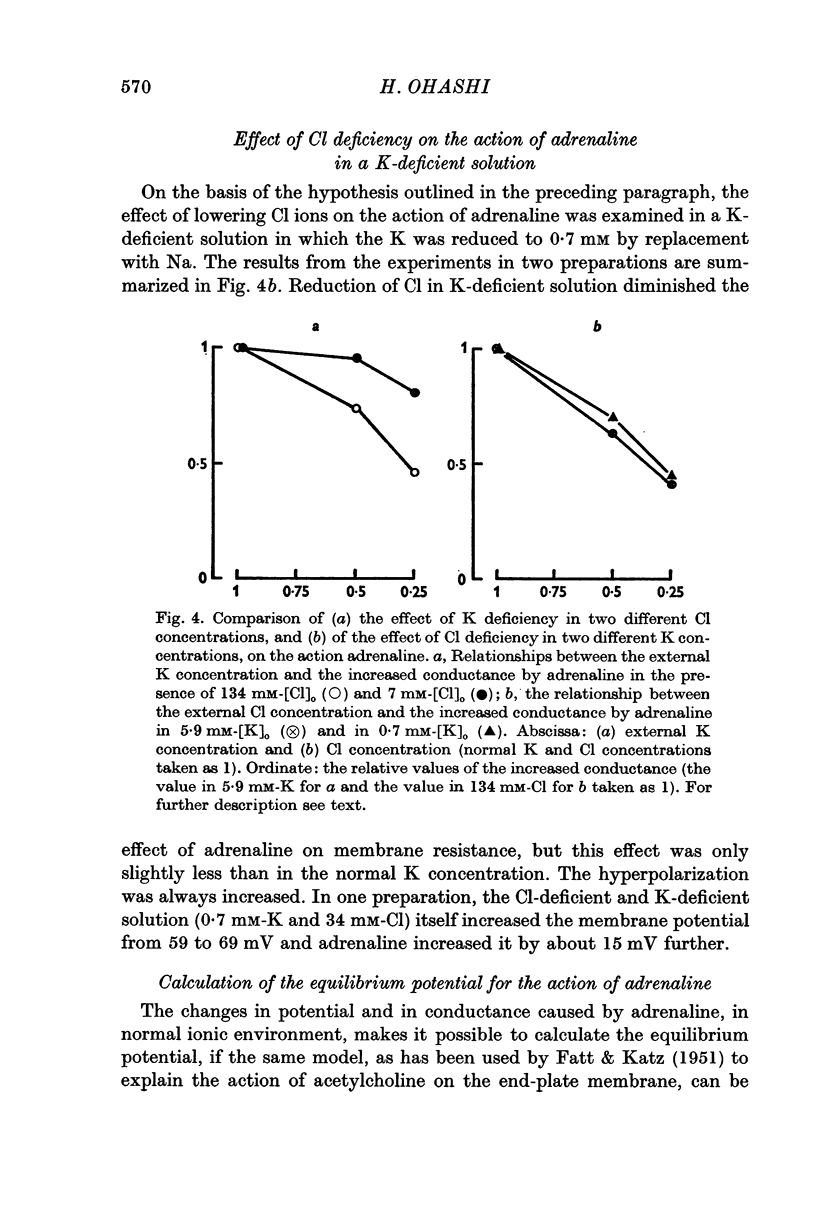
3. The magnitude of the reduction of the electrotonic potential caused by adrenaline was decreased as the concentration of K or Cl ions in the medium was reduced by substituting NaCl or sucrose for KCl or an impermeant anion, benzene sulphonate, for Cl ions. The adrenaline effect remained unaltered when the NaCl was replaced with Tris-Cl.
4. It is therefore concluded that adrenaline opens the ion pathways mainly for K and Cl.
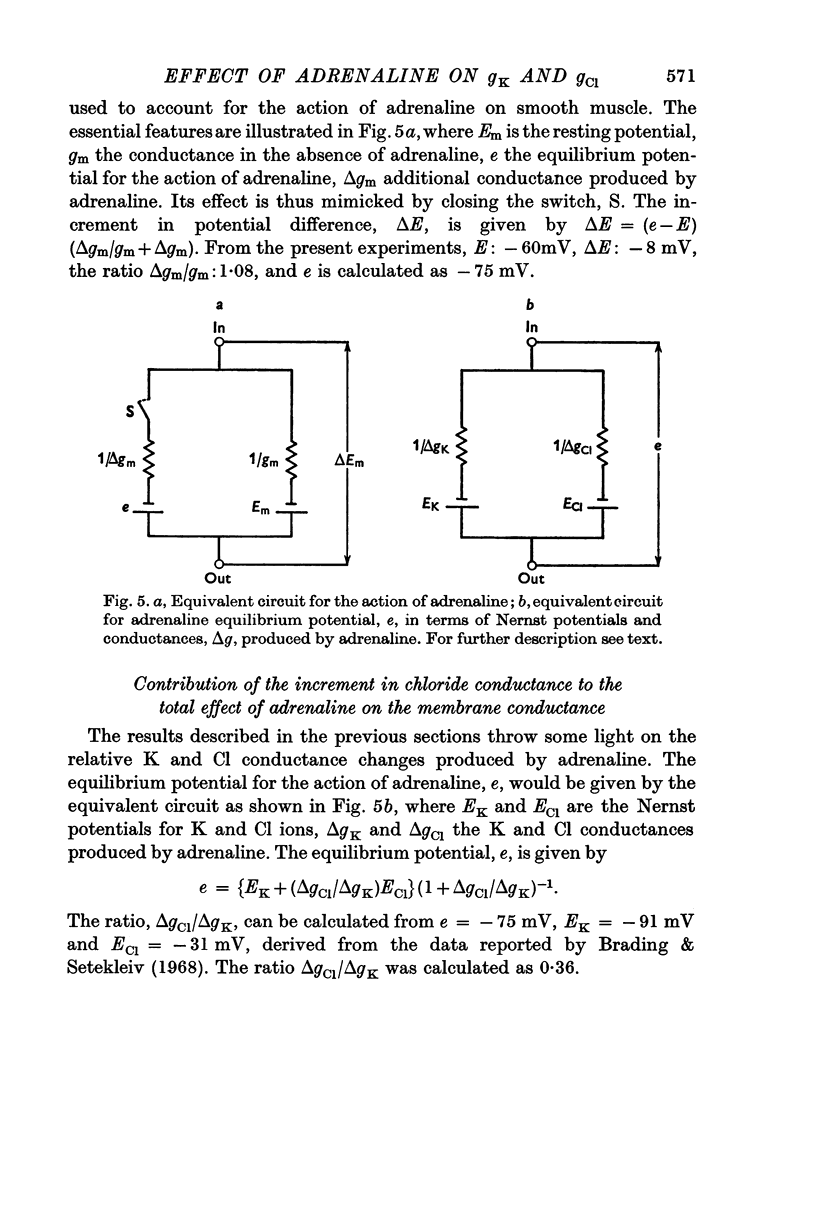
5. On the basis of the changes in potential and in conductance caused by adrenaline, the equilibrium potential for its action was calculated as - 75 mV.
6. The ratio of the Cl component to the K component of the additional conductance increase was obtained as 0·36 using the calculated equilibrium potential and the Nernst potentials for K (-91 mV) and Cl (-31 mV).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in ionic environment on the action of acetylcholine and adrenaline on the smooth muscle cells of guinea-pig taenia coli. J Physiol. 1963 Apr;166:59–74. doi: 10.1113/jphysiol.1963.sp007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The action of adrenaline on excitability and membrane potential in the taenia coli of the guinea-pig and the effect of DNP on this action and on the action of acetylcholine. J Physiol. 1958 Aug 29;143(1):183–194. doi: 10.1113/jphysiol.1958.sp006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Setekleiv J. The effect of hypo- and hypertonic solutions on volume and ion distribution of smooth muscle of guinea-pig taenia coli. J Physiol. 1968 Mar;195(1):107–118. doi: 10.1113/jphysiol.1968.sp008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Tomita T. Effect of anions on the volume of smooth muscle. Nature. 1968 Apr 20;218(5138):276–277. doi: 10.1038/218276a0. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Goodford P. J., Setekleiv J. The action of adrenaline on the ionic content and on sodium and potassium movements in the smooth muscle of the guinea-pig taenia coli. Br J Pharmacol Chemother. 1966 Dec;28(3):296–307. doi: 10.1111/j.1476-5381.1966.tb01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H., MORTON I. K. EFFECTS OF NORADRENALINE AND ISOPRENALINE ON THE PERMEABILITY OF DEPOLARIZED INTESTINAL SMOOTH MUSCLE TO INORGANIC IONS. Nature. 1965 Jan 30;205:505–506. doi: 10.1038/205505a0. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The effect of noradrenaline on the permeability of depolarized intestinal smooth muscle to inorganic ions. J Physiol. 1967 Feb;188(3):373–386. doi: 10.1113/jphysiol.1967.sp008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Electrophysiological study of the intestinal smooth muscle of the guinea-pig. J Physiol. 1967 Jul;191(2):239–255. doi: 10.1113/jphysiol.1967.sp008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H. An estimate of the proportion of the resting membrane conductance of the smooth muscle of guinea-pog taenia coli attributable to chloride. J Physiol. 1970 Sep;210(2):405–419. doi: 10.1113/jphysiol.1970.sp009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]


