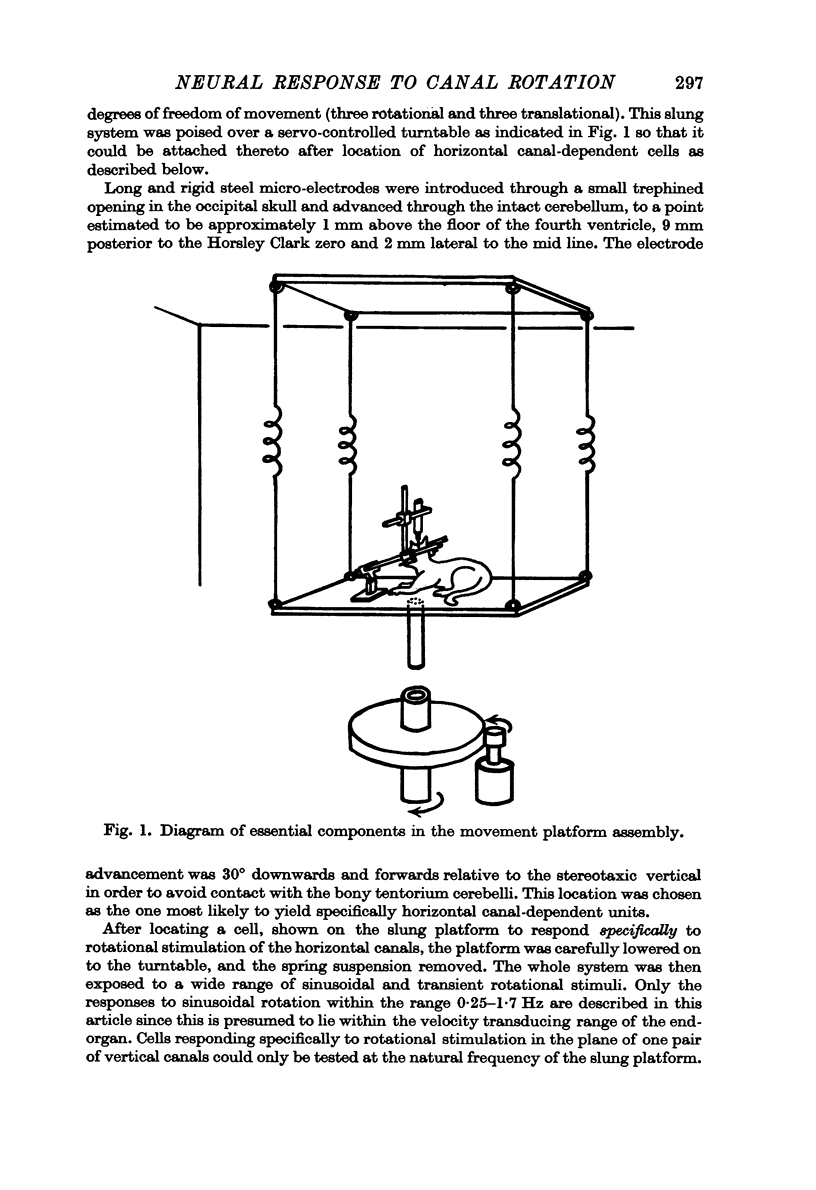
Abstract
1. The characteristics of the dynamic response of specifically canal-dependent neural units in cat vestibular nuclei have been examined during sinusoidal rotation of the head in decerebrate cats over the narrow frequency range 0·-1·7 Hz.
2. Unit action potential frequencies were averaged on line from extracellular steel micro-electrodes stereotaxically located in the vestibular nuclei through the intact cerebellum.
3. Action potential frequency was approximately in phase with stimulus angular velocity, the mean phase for forty-six units being + 11·° S.E. of the mean ± 2·2°). Correction for a form of dynamic asymmetry reduced this value almost to zero.
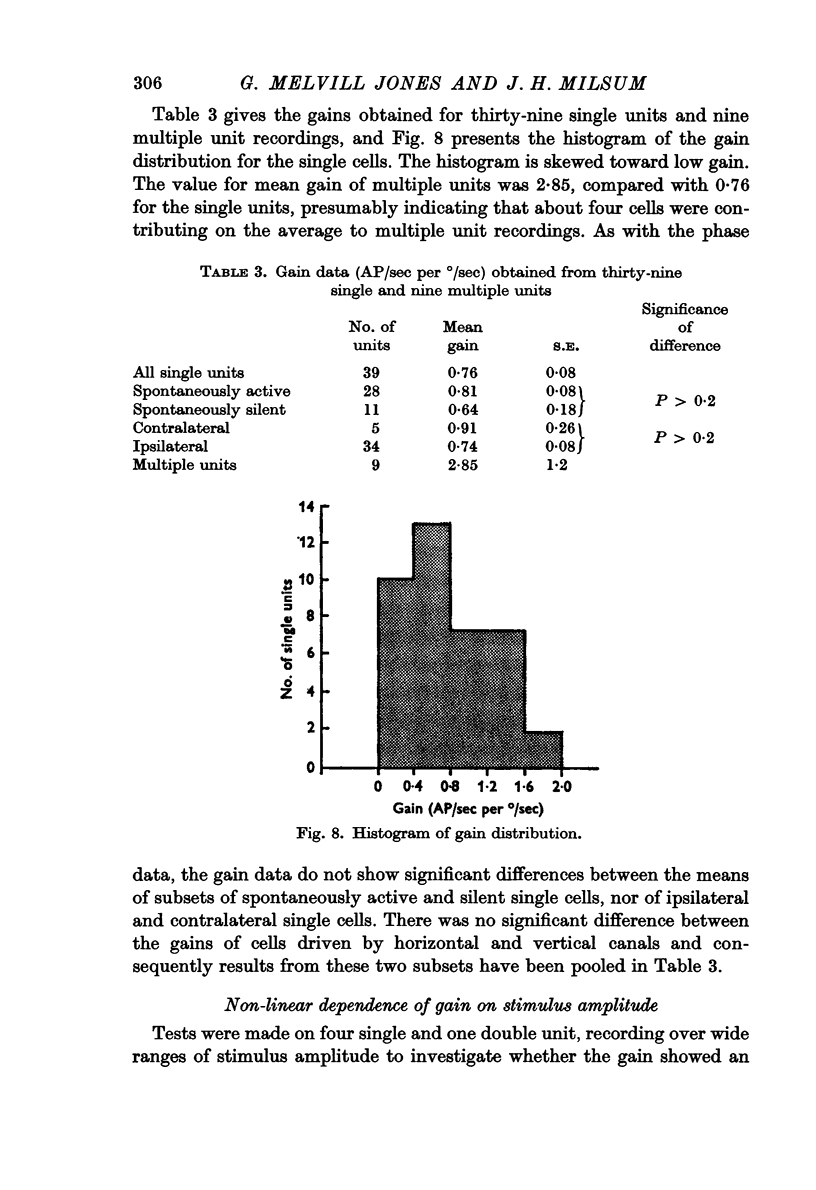
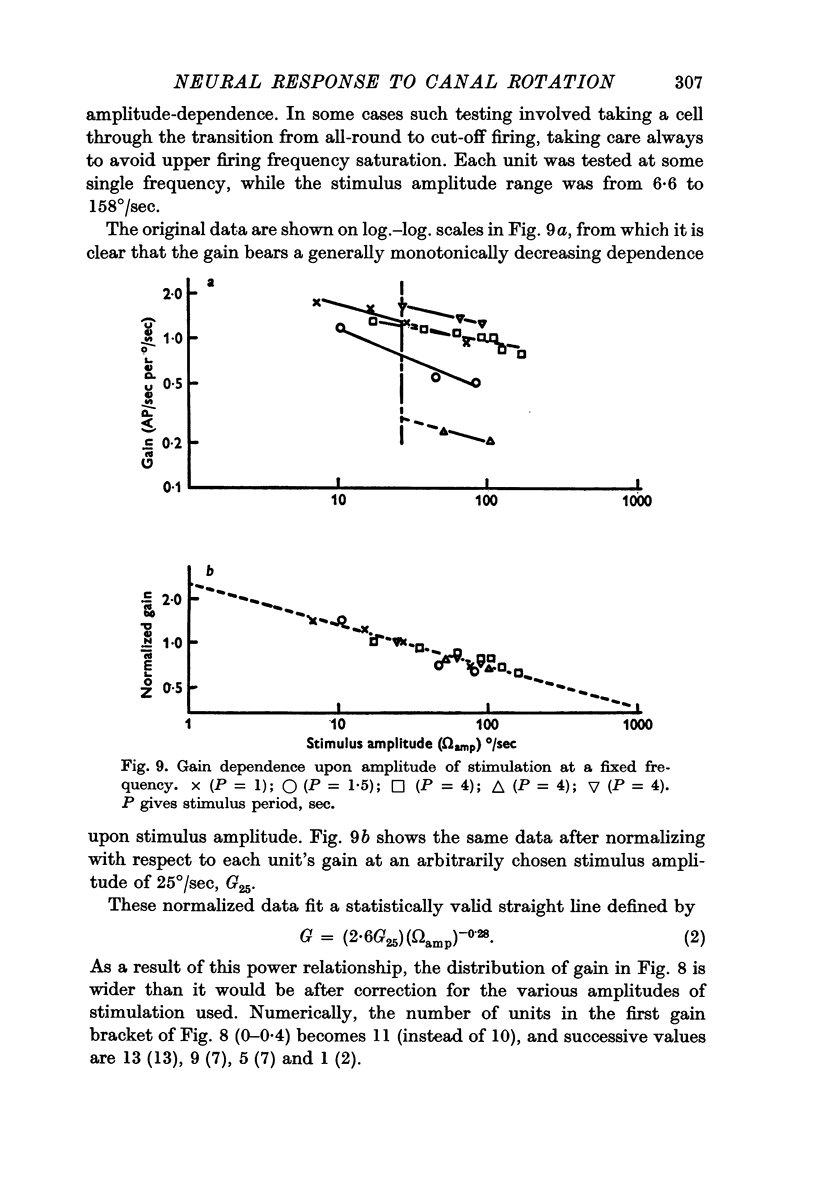
4. The mean gain of thirty-nine single unit responses was 0·76 S.E. ± 0·08) AP/sec per °/sec. The gain varied as the (-0·28) power of stimulus angular velocity, for the five cells appropriately tested.
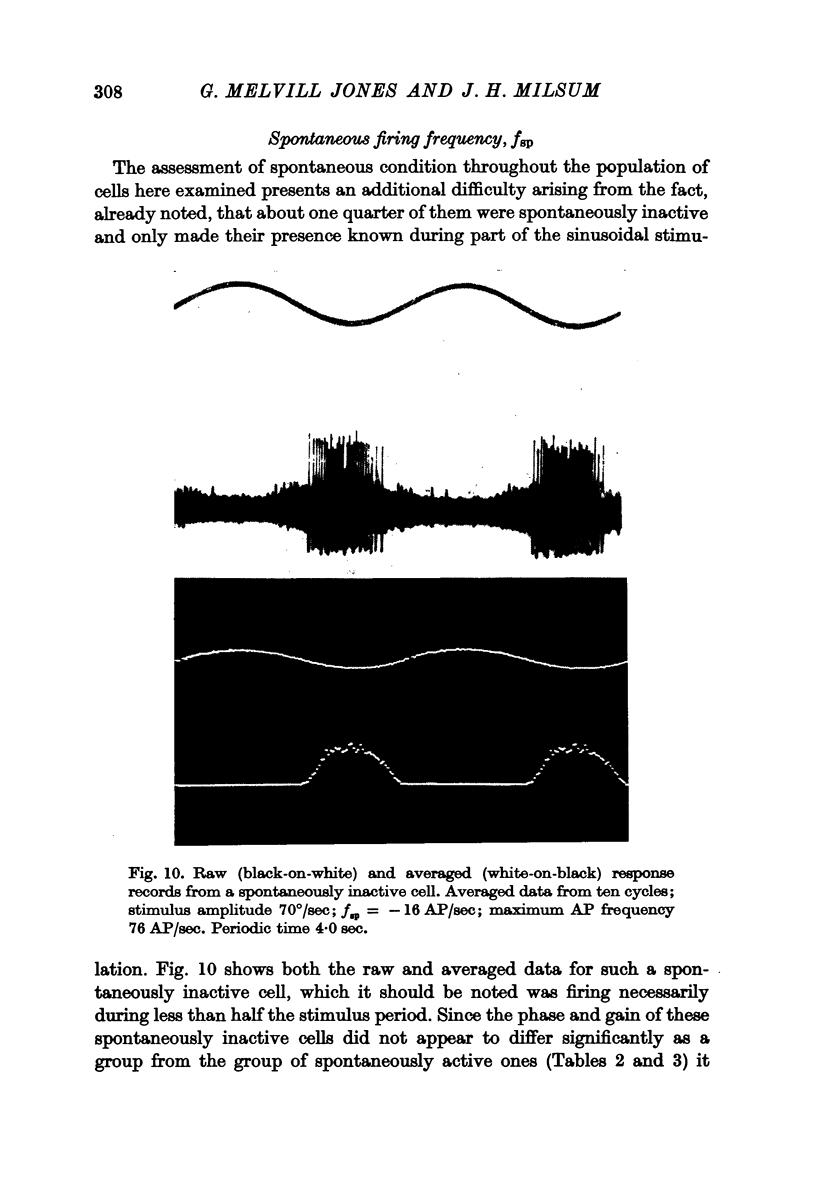
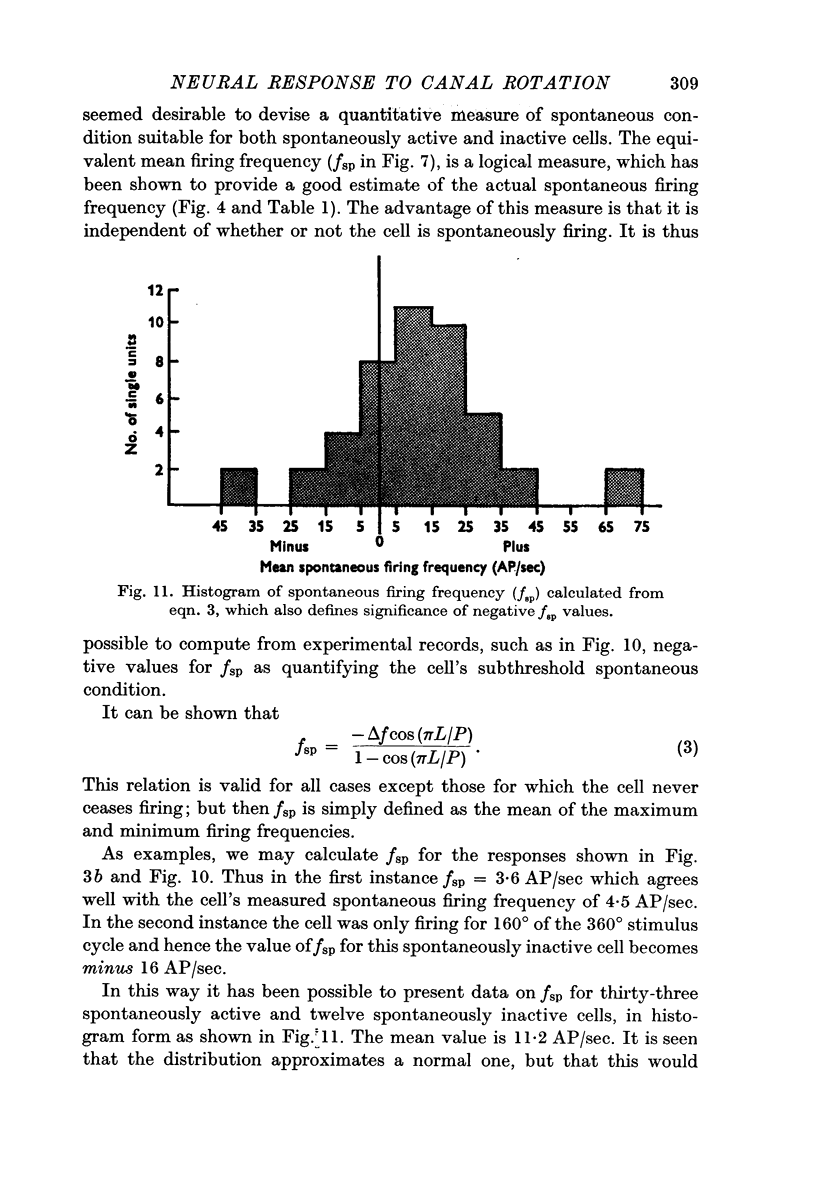
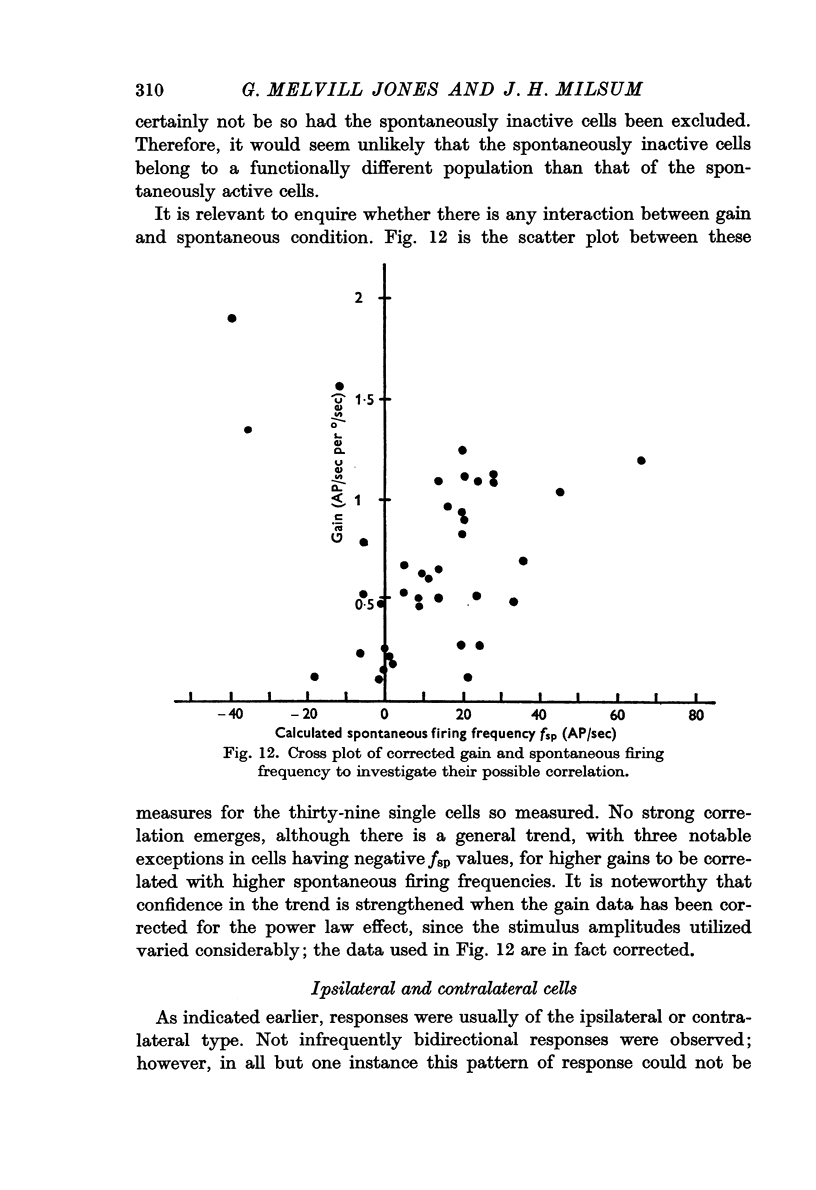
5. A method was evolved for computing the spontaneous condition of cells, in terms of an equivalent spontaneous firing frequency, fsp irrespective of whether they were spontaneously active or silent. This fsp value was normally distributed about a mean value of 11·2 AP/sec (range + 70 to - 40 AP/sec).
6. Directionality was examined in 116 units, of which 62% were ipsilateral (e.g. cells on left side excited by left-going rotational velocity) and 38% contralateral. Ipsilateral units proved easier to isolate and retain than contralateral ones.
7. No significant differences in mean phase or gain were found in the sub-sets of spontaneously active/inactive cells and ipsilateral/contralateral cells.
8. It is inferred that the cell population examined was a functionally homogeneous one in which the neural signal was closely tied to the angular velocity of canal rotation for the narrow band of sinusoidal rotational stimuli here employed. It is suggested that this signal is probably retained essentially intact in the ensemble neural message fed forwards from this region.
Full text
PDF


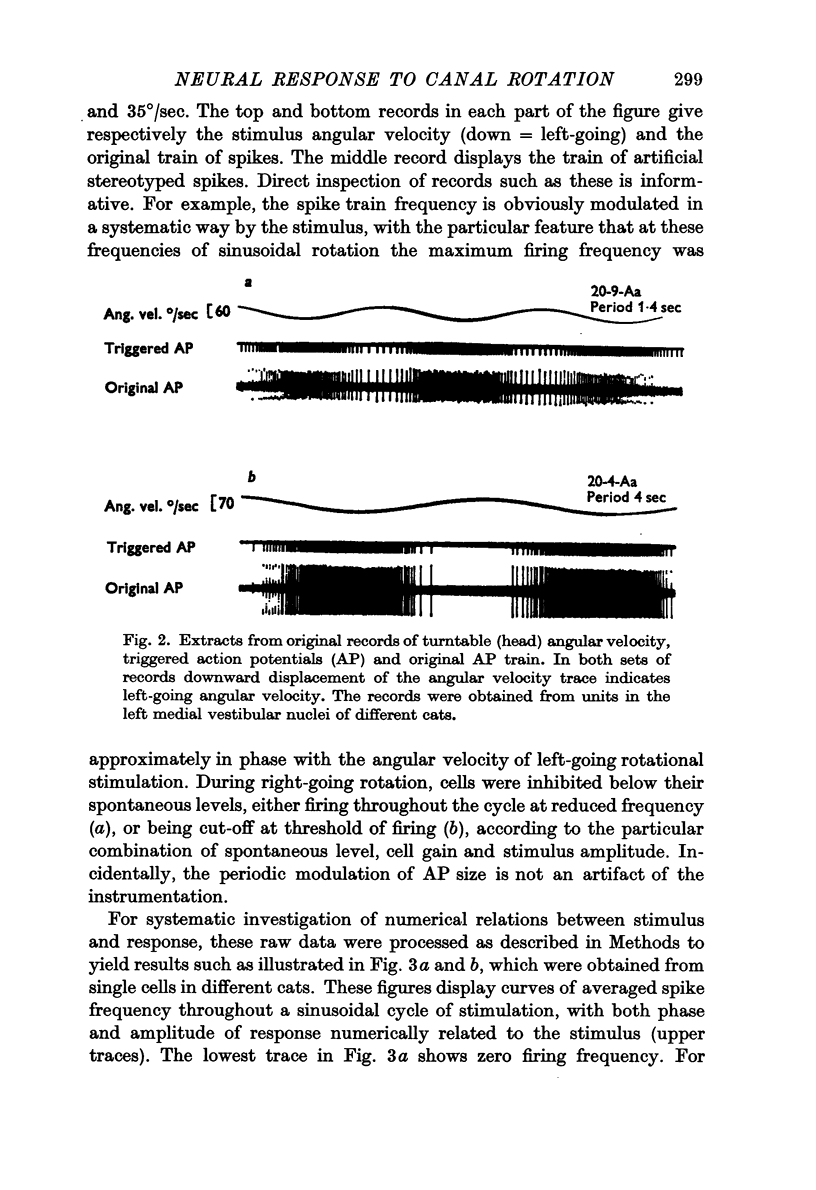
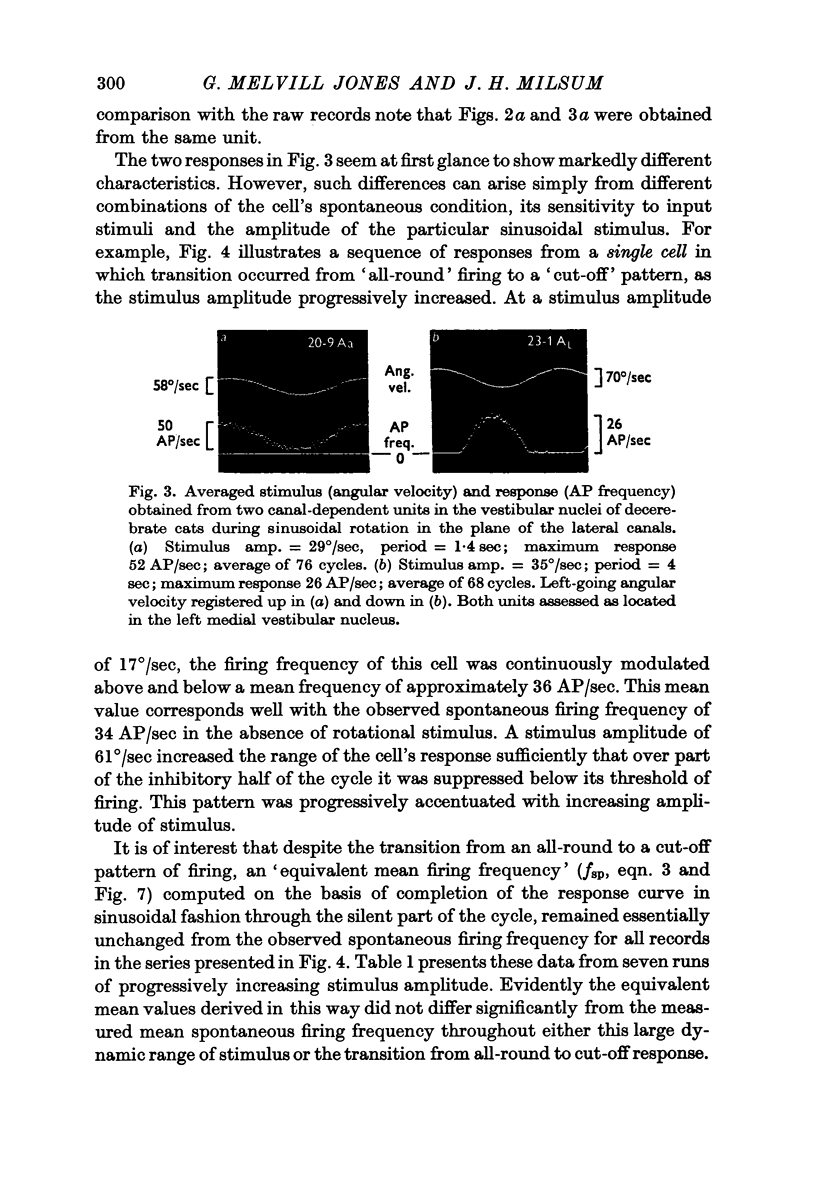







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. Discharges from vestibular receptors in the cat. J Physiol. 1943 Mar 25;101(4):389–407. doi: 10.1113/jphysiol.1943.sp003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández C., Valentinuzzi M. A study on the biophysical characteristics of the cat labyrinth. Acta Otolaryngol. 1968 Mar;65(3):293–310. doi: 10.3109/00016486809120969. [DOI] [PubMed] [Google Scholar]
- GROEN J. J., LOWENSTEIN O., VENDRIK J. H. The mechanical analysis of the responses from the end-organs of the horizontal semicircular canal in the isolated elasmobranch labyrinth. J Physiol. 1952 Jul;117(3):329–346. doi: 10.1113/jphysiol.1952.sp004752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES G. M., SPELLS K. E. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond B Biol Sci. 1963 Mar 26;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Spatial and dynamic aspects of visual fixation. IEEE Trans Biomed Eng. 1965 Apr;12(2):54–62. doi: 10.1109/tbme.1965.4502350. [DOI] [PubMed] [Google Scholar]
- MAYNE R. The dynamic characteristics of the semicircular canals. J Comp Physiol Psychol. 1950 Aug;43(4):309–319. doi: 10.1037/h0054827. [DOI] [PubMed] [Google Scholar]
- Milsum J. H., Jones G. M. Dynamic asymmetry in neural components of the vestibular system. Ann N Y Acad Sci. 1969 Apr 21;156(2):851–871. doi: 10.1111/j.1749-6632.1969.tb14018.x. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Terzuolo C. A. Myotatic reflex: its input-output relation. Science. 1968 Feb 16;159(3816):743–745. doi: 10.1126/science.159.3816.743. [DOI] [PubMed] [Google Scholar]
- Shimazu H., Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966 May;29(3):467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Shimazu H., Precht W. Tonic and kinetic responses of cat's vestibular neurons to horizontal angular acceleration. J Neurophysiol. 1965 Nov;28(6):991–1013. doi: 10.1152/jn.1965.28.6.991. [DOI] [PubMed] [Google Scholar]
- Taylor A. A technique for recording normal jaw movements in conscious cats. Med Biol Eng. 1969 Jan;7(1):89–90. doi: 10.1007/BF02474674. [DOI] [PubMed] [Google Scholar]
- VAN EGMOND A. A. J., GROEN J. J., JONGKEES L. B. W. The mechanics of the semicircular canal. J Physiol. 1949 Dec 15;110(1-2):1–17. doi: 10.1113/jphysiol.1949.sp004416. [DOI] [PMC free article] [PubMed] [Google Scholar]