Abstract
Context
Infertility and pregnancy.
Objective
To assess the effectiveness of site-specific manual soft tissue therapy in (1) facilitating natural fertility and (2) improving in vitro fertilization (IVF) pregnancy rates in women with histories indicating abdominopelvic adhesion formation.
Design and Intervention
Pursuant to 2 promising pilot studies, 53 infertile, premenopausal patients received a 10- to 20-hour series of site-specific manual physical therapy treatments. Seventeen patients hoped to achieve a natural pregnancy; 36 planned to undergo IVF within 15 months. The primary criteria for inclusion in the studies were the inability to conceive following a minimum of 12 months of unprotected intercourse and suspected or confirmed pelvic adhesions due to abdominal and/or pelvic surgery, infectious or inflammatory disease (eg, endometriosis, PID), or trauma. Treatments were specifically designed to address biomechanical dysfunctions of the pelvis, sacrum, and coccyx and restricted soft tissue and visceral mobility due to adhesions or microadhesions affecting the reproductive organs and adjacent structures.
Main Outcome Measures
(1) Natural fertility group: pregnancy within 1 year of therapy and subsequent full-term delivery; (2) Pre-IVF group: pregnancy (via transfer of fresh embryos from nondonor eggs) within 15 months of the last manual treatment date.
Results
Natural Fertility Group
Of the 14 patients available for follow-up (ages 25 to 44; mean, 33.5 years), 10 (71.4%) became pregnant within 1 year, and 9 (64.3%) reported full-term deliveries. Three of the 9 women who delivered reported a subsequent pregnancy, suggesting that the treatment protocol might have lasting effects. Two women have had a second live birth delivery; and the third is still pregnant.
Pre-IVF group
Of the 25 patients available for follow-up (ages 28 to 44; mean, 36 years), clinical pregnancies were documented in 22 of 33 embryo transfers vs the US Centers for Disease Control and Prevention (CDC) 2001 age-adjusted expected number of 12.7 (P < .001). The estimated odds ratio for a successful pregnancy in a cycle (manual treatment: no treatment) is 3.20 (95% confidence interval = 1.55–8.4).
Conclusions
The data trend across these studies suggests that this innovative site-specific protocol of manual soft-tissue therapy facilitates fertility in women with a wide array of adhesion-related infertility and biomechanical reproductive organ dysfunction. The therapy, designed to improve function by restoring visceral, osseous, and soft-tissue mobility, is a nonsurgical, noninvasive manual technique with no risks and few, if any, adverse side effects or complications. As such, it should be considered a new adjunct to existing medical infertility treatments.
Introduction
The purpose of the present series of investigations was to assess the effectiveness of site-specific manual soft-tissue therapy in treating infertility in women with a history indicating probable abdominopelvic adhesion formation, eg, prior surgery, endometriosis, infection, inflammatory process, trauma, or tubal obstruction.
Adhesions and Infertility
In the United States, infertility is defined as the inability to conceive after 12 months of unprotected sexual intercourse.[1,2] Internationally, the time frame is generally longer — 24 months.[3] Infertility is a common problem affecting 10% to 15% of heterosexual couples. Estimates suggest that 40% of the problems are attributable to the female, 40% to the male, 20% to both or unknown, and that some 25% of infertile couples have > 1 factor impeding fertility.[3] As most infertility research lacks control couples for comparison, much of the infertility literature is anecdotal.[4]
Of the approximately 5 million infertile women in the United States, it is estimated that 2 million (40%) have medical or hormonal infertility; 1 million (20%) have idiopathic infertility; and 2 million (40%) have mechanical infertility.[5]
Pelvic adhesions are often cited among the primary causes of mechanical infertility.[4,6] Adhesions are deposits of fibrous tissue that form as a natural inflammatory response to tissue damage after surgery, infection, inflammation, or trauma. They form as a by-product of the healing process and may remain long after the original site of inflammation or trauma has healed. They may adhere to a specific organ or muscle, either within the myofascial structure of the organ, on its surface, or as an attachment to neighboring structures. Wherever they occur, adhesions distort the anatomy and cause decreased mobility and function.[4]
In addition to being a common outcome of pelvic surgery, the formation of pelvic adhesions is known to accompany related conditions such as endometriosis, pelvic inflammatory disease (PID), tubal obstruction, polyps, pelvic spasms, bowel obstruction, and chronic abdominopelvic pain.[6–8] It is presumed that some of these dysfunctions cause, or are caused by, adhesions. Moreover, a certain proportion of idiopathic infertility may be due to microadhesions that have formed in the pelvis as the body healed from a previous inflammation or trauma. Microadhesions are often too small to see, and thus difficult to diagnose.
Effects of Abdominopelvic Adhesions
In sum, adhesions can restrict the mobility and function of the organs, ligaments, osseous structures, muscles, fascia, and nerves. Thus, they affect the biomechanics of the entire abdominopelvic region, limiting the ability to conceive even with in vitro fertilization (IVF) and other assisted reproductive technologies (ART).
Infertility-causing adhesions may form in the following locations:
on uterine walls and ligaments, increasing the possibility of uterine spasm, implantation problems, and miscarriage and decreasing the ability to conceive;
at and within the tissues of the cervix, creating stenosis, affecting the relaxed midline position, contributing to uterine spasms, and complicating sperm transfer to the uterus;
on the surface of the ovaries, preventing exposure of the ovum and making transfer to the fallopian tube difficult;
at the distal aspect of the fallopian tube, restricting the tentacle-like grasping of the egg by the fimbria, hence increasing its risk of being wasted in the abdominal cavity; and
anywhere on the inside or outside of the fallopian tube, causing partial or total tubal occlusion, decreasing the probability of conception, and increasing the chance of an ectopic pregnancy.[9–11]
Value of Intervention
Clinically, we have observed that site-specific manual soft-tissue therapy improves soft-tissue mobility, elasticity, and distensibility. Theoretically, mobilization of the soft tissue may break collagenous cross-links and adhesions that cause pain and dysfunction,[12] including physician-diagnosed mechanical infertility.
In addition to its apparent use as a natural infertility treatment, this therapy functions as an adjunct to regular gynecologic care when applied before intrauterine insemination (IUI) and IVF. We suspect that the therapy specifically helps improve mobility and motility of the reproductive organs by decreasing the following:
adhesions and microadhesions on and within the uterine walls, helping to create a more hospitable surface for implantation;
uterine and cervical hypertonicity and spasm, thus creating a more relaxed environment for implantation;
cervical stenosis, adhesions, and tensions within the cervix and its attachments, thus improving cervical mobility and facilitating transfer to the preferred implantation site.
Connective Tissue and Adhesions
The ability of manual therapy to affect connective tissues and adhesions has support in the basic literature on mechanical tissue testing and connective tissue physiology and remodeling. Specific sustained physical forces applied to a given area alter connective tissue length and mobility.[13]
Adhesion formation occurs after soft-tissue trauma and is caused by an inflammatory response to tissue damage. The body responds to injury by activating macrophages to debride and clean the damaged area. Fibroblasts begin to replace lost collagen, producing a fibrinous exudate. Myofibroblasts then appear, anchor to adjacent collagen fibers, and contract, thus shrinking the tissue.[14–17]
As collagenous fibroblasts align within the structure, collagen is laid down in a haphazard manner, and cross-links begin to form. The result is the formation of a fibrinous adhesion, which may cause a subsequent adherence of the adjacent serosal surfaces. Tissue shrinkage leads to dysfunctional movement of the area that, in turn, creates more mechanical irritation, thus perpetuating the cycle.[14–17] These mechanical components have been proposed as the underlying mechanism of adhesion-related pain.[18]
As healing time increases, cross-links may grow into microadhesions, then adhesions, and eventually thicken into scars.[15,16] Minor adhesion formations are often limited and may be absorbed within a few days by fibrinolytic mechanisms, but permanent adhesions can form between the peritoneum and the serosal surface of adjacent organs. These permanent adhesions are considered a pathologic state in which the continuous unity of the peritoneal wall or serosal surface of organs is destroyed, leading to impairment of their physiologic functions.[19,20] Mobilization of the soft tissues using site-specific manual therapy appears to break the attachments of the collagenous cross-links within the adhesions, thus restoring normal mobility and function to the previously adhered organs.
Physical Therapy and Infertility
A search into the use of manual physical therapy as an infertility treatment yielded a series of studies conducted between 1978 and 1989 in the Czech Republic. The Mojzisovà method includes a combination of soft tissue and osseous mobilization techniques, post-isometric relaxation, and a home exercise program over a 6-month treatment period. It is based on the premise that accidents (including falls) and sedentary lifestyles can cause blockages or constrictions in the lower spine that lead to pelvic spasms and other functional disturbances of the pelvic region. Thus, according to Mojzisovà, “there is a direct relationship between the condition of the lower back muscles and the way the reproductive organs function.”[21,22]
The purpose of the second Prague study, based on 2006 randomly selected infertile women, was to determine which types of infertility were best suited for treatment by the Mojzisovà method. Results showed that conception rates ranged from a low of 11% for women aged 40 to 44 to a high of 46% for the age group 20 to 24. Other factors increasing the chance of success included an active lifestyle and the absence of tubal obstructions and other intrusive conditions, such as PID, abdominal and/or pelvic surgery, and ectopic pregnancy.[21]
A subsequent study (1987 to 1989) based on the above findings compared the Mojzisovà method with several control treatments. Criteria for participation were as follows: (1) age between 22 and 30 years; (2) normal quality/quantity of partner's sperm; and (3) patency of fallopian tubes. The study population included 166 women whose mean duration of infertility was 4 years; 118 women completed the trial. The mean conception rate for the main experimental group was significantly higher than that for the 3 control groups, who either performed “non-genuine” exercises or did not exercise at all - 34.3% (12/35) vs 8.4% (7/83) (P < .01).[22]
Pilot Studies
Pilot Study #1 (1989 to 1992)
Facilitating fertility through this site-specific soft-tissue therapy originated as an unplanned outcome of treating physical therapy patients for a variety of pelvic pain symptoms in areas where decreased tissue mobility was noted. In brief, 4 previously infertile women became pregnant coincidental with their treatment. Two of the women, aged 28 and 42, reported infertility as a result of bilateral tubal occlusion; they had been trying to conceive for 7 and 10 years, respectively. Their treatment protocols had been designed to decrease pain and increase function by breaking adhesive cross-links at specific sites in the abdominal and pelvic regions of the body. All 4 pregnancies resulted in full-term deliveries, and 1 woman reported a subsequent full-term pregnancy and live birth. As a retrospective review of these cases, documented through clinical observation, patient reports, and gynecologic records, Pilot Study #1 was the first test of the hypothesis that the therapy could facilitate fertility in previously infertile women.[23]
Pilot Study #2 (1995 to 1997)
In a delayed attempt to substantiate the results of Pilot Study #1, a prospective study with 4 new patients was conducted. To test the hypothesis that the therapy could decrease adhesions and therefore improve reproductive organ function, Pilot Study #2 required bilateral tubal occlusion, diagnosed by pre- and posttreatment hysterosalpingogram (HSG), laparoscopy and/or laparotomy. Although 2 patients showed no change in patency after treatment, the third patient exhibited 1 patent tube, and the fourth demonstrated 1 patent tube and 1 improved tube.[23]
Figures 1 and 2 depict pretreatment and posttreatment HSGs for a 34-year-old woman with no prior pregnancies who had been infertile for 8 years. She was referred to physical therapy with a history of bilateral occlusion with hydrosalpinx, as diagnosed by chromotubation during laparoscopy and laparotomy. Further support for this diagnosis was provided by 2 separate pretreatment HSG studies approximately 1 year apart. In a posttreatment HSG, 1 tube demonstrated free spillage of contrast dye, and the contralateral tube was improved with increased migration of the dye (ie, the contrast medium filled more of the ampullary portion of the contralateral tube).[23]
Figure 1.
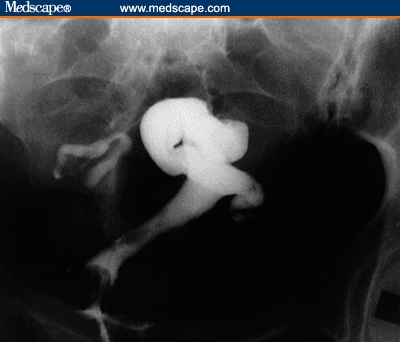
Pretreatment HSG for a 34-year-old woman: bilateral tubal occlusion with left hydrosalpinx. Diagnosis was consistent with pretreatment laparoscopy and laparotomy.
Figure 2.
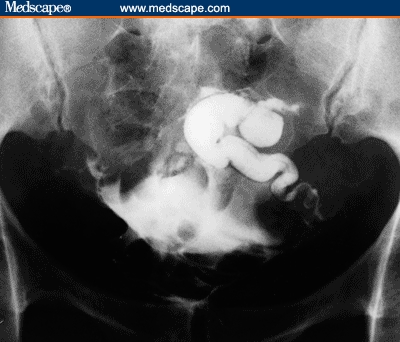
Posttreatment HSG: persistent hydrosalpinx with increased migration of the dye in the left tube; free spillage of contrast via the right tube.
The promising results obtained in Pilot Studies #1 and #2 suggested the methodology for the 2 subsequent studies included in this article: I. Facilitating Natural Fertility, and II. Improving IVF Pregnancy Rates.
Studies I and II (1998 to 2003)
Although each study is separately presented below, many subject characteristics and the intervention itself are common to both. Patient histories were obtained from medical records and included physical therapy and biomechanical assessments; gynecologic, surgical and trauma histories; and prior infertility tests, diagnoses, and treatments.
Subject Selection
Presence of Adhesions
The purpose of the 2 studies was to assess the effectiveness of site-specific manual soft-tissue therapy in treating biomechanical infertility in women with probable abdominopelvic adhesion formation. Thus, all enrolled subjects had histories of conditions indicating a strong probability of adhesion formation before treatment (ie, abdominal and/or pelvic surgery, infectious or inflammatory disease, or trauma). Moreover, 48.7% of patients had definite diagnoses of “adhesions” affecting the reproductive and/or neighboring structures. Although it seemed unlikely that manual soft-tissue therapy would have a direct effect on patients also having medical or hormonal infertility, no patient was excluded from the studies for these conditions.
Medical Histories
The relevant medical histories for the subjects in the 2 studies include the following:
Gynecologic: Abdominopelvic pain, abortion, adhered ovaries at fimbriae, adhesions (abdominal, pelvic), bicornuate uterus, bladder infection, C-section, chronic pelvic inflammation, chlamydia, cystitis, D&C, dysmenorrhea, dyspareunia, ectopic pregnancy, endometriosis, failure to ovulate, fibroids, hydatid cyst of Morgagni at tube, hydrosalpinx, interstitial cystitis, irregular menstrual periods, multiple miscarriage, partially blocked and adhered tubes, numbness at C-section scar, ovarian cysts, PID, pelvic scarring, polyps in uterine horn, ruptured cyst, thyroid and hormonal problems, uterine prolapse, tubal occlusion (unilateral, bilateral), tubal phimosis, urinary incontinence, and vaginitis.
Surgical: Abdominal, abortion, appendectomy, bladder repair, C-section, cervical, D&C, episiotomy, fibroidectomy, hysteroscopy, laparoscopy, laparotomy, lysis of adhesions, myomectomy, ovarian cystectomy, pelvic, tuboplasty, and uterine suspension.
Trauma: Broken bones; falls; injuries to low back, hip, pelvis, sacrum, and coccyx; car accidents; and physical and sexual abuse.
Prior infertility tests and diagnoses: Infertility tests included gynecologic physical examinations and cultures, FSH and TSH tests, ultrasound, HSG, laparoscopy, and laparotomy. Some patients also had hysteroscopy, endometrial and peritoneal biopsies. Infertility diagnoses included hormonal problems, total bilateral occlusion, unilateral occlusion with contralateral tube partially blocked, and hydrosalpinx.
Prior infertility treatments: In addition to HSG, laparotomy, laparoscopy, and hysteroscopy (used primarily for diagnosis), prior infertility treatments included surgery (see above); pharmaceuticals (ie, clomiphene [Clomid] , estradiol, FSH, gonadatropins [Lupron], menotropin [Repronex], micronized progesterone [Prometrium]); and assisted reproductive techniques (ie, IUI and IVF).
The Intervention
The primary goals of manual therapy are to decrease pain and restore mobility. The intent of the particular therapy used in this study is to create microfailure of collagenous cross-links, the “building blocks” of adhesions. These unique soft-tissue techniques were developed after extensive study of current, innovative physical therapy methods.
Following a thorough medical, gynecologic, and surgical history, specific sites of visceral cross-linking were deduced as likely adhesion sites. The therapist also employed sufficient palpation and evaluation skills to note areas of decreased mobility. The restricted soft tissues were engaged and cross-links were perceived to release as evidenced by increased mobility at the precise sites of visceral and myofascial restrictions after each therapy session.
After a perceived increase in histologic length (presumably due to deformation of collagenous cross-links), the soft tissues were noted to become more pliable, with increased mobility and flexibility. These changes were further demonstrated by improved alignment, biomechanics, and increased range of motion of osseous and soft-tissue structures. Many patients reported a decrease in pain symptoms, presumably as a result of decreased pressure on nerves and pain-sensitive structures.
In accord with the standards of the American Physical Therapy Association, detailed clinical records were kept of each patient's visit, including treatment dates and duration, symptomatic complaints, areas treated, and treatment techniques performed.[24] Depending on the patient's schedule and geographic location, the frequency and duration of treatment ranged from a 1-hour session at weekly or longer intervals to intensive sessions of 2 to 4 hours of treatment daily, performed over 5 days. The standard length of the therapy sessions was 1 to 2 hours, minus 15 minutes for room preparation and paperwork.
Study I. Treating Female Infertility With a Manual Physical Therapy Technique
Subjects
Selection
The primary criteria for inclusion in this prospective study were (1) the inability to conceive following at least 12 months of unprotected intercourse, and (2) suspected or confirmed pelvic adhesions attributed to abdominal and/or pelvic surgery, infectious or inflammatory disease (eg, endometriosis, PID), miscarriage, or trauma within the abdominopelvic area. A total of 17 women were selected to receive a series of site-specific manual physical therapy treatments; 3 were lost to follow-up.
Gynecologic History
All 14 patients in this study had proven or clinically well-supported suspicion of adhesions. Medical diagnoses included:
| Infectious/inflammatory disease | (13) | 92.8% |
| Abdominopelvic trauma | (12) | 85.7% |
| Abdominopelvic surgery | (11) | 78.6% |
| Endometriosis | (7) | 50.0% |
| Confirmed pelvic adhesions | (5) | 35.7% |
| Pelvic inflammatory disease | (2) | 14.3% |
Characteristics
Study participants were a multiethnic, primarily white group, ranging in age from 25 to 44 years. The mean age was 33.5 (median, 32); and duration of infertility ranged from 1 to 20 years, with a mean of 4.9 (median, 4) years.
Procedures/Intervention
Largely on the basis of standard physical therapy practices, completion of a minimum of 20 treatment hours (or pregnancy during the course of therapy) was one of the few criteria for inclusion in the study.[24] None of the patients received concurrent infertility therapies during the treatment period.
Data Collection
Study patients were evaluated and treated between May 1998 and February 2002 and tracked for at least 1 year following therapy. This does not imply that failure to become pregnant within a year was deemed permanent infertility,[4] but in terms of facilitating fertility in a timely manner, 1 year sufficed for outcome assessment. Patients who became pregnant during treatment were tracked through expected delivery date. Follow-up data were obtained via questionnaires, telephone calls, letters, and email.
The final data set includes 14 patients who completed the recommended 20 hours of therapy or else became pregnant before completing therapy. Three patients were omitted because they did not respond to follow-up attempts.
Results
For purposes of evaluating the effectiveness of site-specific manual soft-tissue therapy in facilitating fertility in women with a history indicating probable abdominopelvic adhesion formation, positive clinical outcomes were defined as (1) natural pregnancy within 1 year of the last treatment date, and (2) subsequent full-term delivery.
The duration of therapy was 1 to 24 weeks; median hours of therapy, 11. Of the 10 subjects who became pregnant, 9 conceived before receiving the full 20 hours of therapy. Having achieved their objective (pregnancy), continued treatment was deemed unnecessary.
As shown in Table 1, 10 of the 14 study participants (71.4%) demonstrated posttreatment pregnancy, and 9 of 14 (64.3%) subsequently delivered a full-term baby. Hence, 90% (9/10) of the women who conceived had a live birth delivery, including 3 patients who had reported unilateral or bilateral tubal occlusion. The one “unsuccessful” patient (age 32) lost her baby at 28 weeks gestation because of umbilical cord strangulation.
Table 1.
Pregnancies and Full-term Births
| N | # Pregnant | # Delivered | % Pregnant | % Delivered |
| 14 | 10 | 9® | 71.4 | 64.3 |
® 9/10 (90%) subjects who conceived had a full-term delivery.
Various studies over the decades have unequivocally demonstrated the statistically significant decline in female fertility with age. Indeed, as one expert claims, “a woman's 35th birthday marks a watershed that irreversibly lowers the probability of reproduction in her life.”[4] The 1987 – 1989 Mojzisovà study (see above) pointedly excluded subjects older than 30 years of age.[22] In view of the import of this factor, Table 2 shows the rate of pregnancy by age range in the current study. Of the patients in the 31 to 45 age range, 63.6% (7/11) conceived compared with 100% (3/3) patients in the 25 to 30 age range.
Table 2.
Pregnancies by Age Range
| Age Range | # of Subjects | # of Pregnancies | % Pregnant |
| 25–30 | 3 | 3 | 100.0 |
| 31–45® | 11 | 7 | 63.6 |
| Total | 14 | 10 | 71.4 |
®The Mojzisovà study excluded women above age 30.
As age 35 is considered the “watershed” for reproductive probability, Table 3 shows the pregnancy and live birth delivery rates obtained by patients in the younger than 35 and 35+ age groups. Of the 9 patients in the < 35 group, 77.8% (7/9) conceived and 66.7% (6/9) delivered, as compared with the 60% (3/5) pregnancy and live birth delivery rates of patients in the 35+ age group.
Table 3.
Pregnancies/Deliveries by Age Group
| Age Group | # of Subjects | % Pregnant (n) | % Delivered (n) |
| Below 35 | 9 | 77.87 (7) | 66.7 (6) |
| 35+ | 5 | 60.0 (3) | 60.0 (3) |
| Total | 14 | 71.4 (10) | 64.3 (9) |
Treatment Safety
None of the patients in the study reported any observable complications or adverse side effects as a result of their treatment. Indeed, whereas all 14 patients presented with pain at their initial evaluation, 13 of the 14 reported decreased pain during or after treatment.
Discussion
Approximately 40% of cases of female infertility are biomechanical and attributable to scarring and/or pelvic adhesions resulting from previous abdominal/pelvic surgery, endometriosis, abdominopelvic infection, inflammatory disease, postinfection tubal damage, ruptured appendix, ruptured ovarian cysts, bowel disease, or foreign body reaction. Clinically, women with known pelvic adhesions and chronic pelvic pain have responded well to this manual physical therapy.
Related Research
Although our results can be compared with those of the Mojzisovà study (1987–1989), the inclusion criteria differed markedly; ie, most of our patients were > 30 years of age, and 4 reported tubal occlusion. Women with these characteristics were specifically excluded from the Mojzisovà study. Moreover, we purposely sought to treat women with other factors known to decrease the chance of positive results, ie, hormone problems, PID, abdominal and/or pelvic surgery, and ectopic pregnancy. Nevertheless, the mean conception rate for the study group was 71.4% vs 34.3% for the group treated by the Mojzisovà method.[22]
Current and Future Research
On the basis of the encouraging results (see above), a number of future studies in facilitating natural fertility are planned. One of these, a virtual replication of the present study, will use a much larger sample of infertile women, with subjects randomized into experimental (treatment) and control (no-treatment and/or pseudo-treatment) groups.
As Pilot Study #2 suggested, this therapy seemed capable of assisting women with occluded fallopian tubes. The present study supported this finding in that 3 of the 4 patients who reported tubal occlusion had live births following therapy, including 1 woman who had been diagnosed (by laparoscopy) with total bilateral occlusion. The therapy also appears efficacious for some women who have had no success with traditional infertility treatments alone, including fertility drugs, IUI, IVF, and other assisted reproduction techniques. Separate investigations in these related areas are now being conducted.
Another area of future investigation is the long-term duration of positive effects. In Study I, 3 patients who delivered following therapy reported a subsequent pregnancy: 2 women have had a second live birth, and the third is still pregnant. In time, it might also be possible to analyze positive outcomes in relation to factors such as specific dysfunctions, pain complaints and resolution, previous miscarriages, primary and secondary infertility, duration of infertility, type and number of prior infertility therapies, prior surgeries, and the optimal number of therapy hours for individual patients.
Lastly, there are strong indications of the efficacy of this therapy as a pre-IVF adjunct, as shown in Study II.
Study II. Improving IVF Pregnancy Rates With a Manual Physical Therapy Technique
Several of our patients who had been receiving the treatment for abdominopelvic pain announced their intention to undergo IVF because they were unable (for various diagnosed causes) to achieve a natural pregnancy. Thus, in 1998, we began investigating the efficacy of site-specific soft-tissue therapy as an adjunct to ART for women with suspected or diagnosed pelvic adhesions.
Introduction
In the United States alone, the number of live birth deliveries per year resulting from all ART procedures has risen exponentially from 5600 in 1991,[25] to 14,573 in 1996, to 29,344 in 2001.[26] Of the 29,344 live birth deliveries, 21,813[27] were through the use of the woman's own (nondonor) fresh eggs or embryos, which accounts for 75.2% of all ART procedures.[28] [Note: A live birth delivery may include multiple babies.[26]]
For those unfamiliar with the process, the typical ART cycle using fresh nondonor eggs or embryos includes 4 prepregnancy steps. The cycle starts when the woman begins taking drugs to stimulate ovulation. If successful, the next step is egg retrieval. The eggs are combined with sperm and a few days after fertilization (if successful), selected embryo(s) are transferred into the uterus. This process is known as IVF and represents 99% of ART procedures.[27,29]
A sobering fact is that the 21,813 live births using fresh nondonor eggs represent only 27% of ART cycles started (80,864); 31.4% of egg retrievals (69,515); and 33.4% of embryo transfers (65,363).[27,30]
For ART data collection purposes, pregnancy is defined as a clinical rather than a chemical pregnancy.[31] Although a chemical pregnancy (positive pregnancy test) can be detected by a positive human chorionic gonadotropin within 5 days, a clinical pregnancy is one that has progressed to the stage where the gestational sac and fetal heart motion can be documented by ultrasound.[25,31]
Given that only 33.4% of embryo transfers result in a live birth, it is not surprising that a priori pregnancy success rates, expressed as pregnancy per cycle, retrieval, or transfer, are also disappointingly low. The 26,550 clinical pregnancies obtained by ART cycles using fresh nondonor eggs or embryos in 2001 represent 32.8% of the total ART cycles started (80,864); 38.2% of egg retrievals (69,515); and 40.6% of embryo transfers (65,363).[27,29]
Although other factors (ie, indication, number of transferred embryos) are involved, the age of the female is the primary determinant of IVF success at every stage of the ART process: the prognosis for women older than age 40 is considerably poorer than those who are younger.[25] For women in the 5 age groups, < 35; 35–37; 38–40; 41–42, and > 42, the calculated 2001 national rates of pregnancies per embryo transfer are, respectively: 48%; 42%; 34%; 24%; and 12%.[32] The corresponding rates for live births per transfer are: 41%; 35%; 25%; 14%; and 6%.[29, 32]
Since live births per embryo transfer have been steadily improving (from 28% in 1996 to 33.4% in 2001),[33] an intervention that increases the frequency of clinical pregnancy rates, particularly in the older age groups, would automatically increase the frequency of live-birth deliveries.
Subjects
Selection
As in Study I, the primary criteria for inclusion in this prospective study were the inability to conceive following at least 12 months of unprotected intercourse and suspected or confirmed pelvic adhesions due to abdominal and/or pelvic surgery, infectious or inflammatory disease (eg, endometriosis, PID), miscarriage, or trauma within the abdominopelvic area. Other criteria were the following:
intention to undergo IVF therapy within 15 months of the last (manual physical therapy) treatment date;
decision to use fresh nondonor (own) embryos;
ability to progress to the embryo transfer stage of the ART procedure (see footnote).
Between September 1998 and January 2003, a total of 36 women received an individualized series of site-specific manual physical therapy treatments. Of these, 11 patients were ultimately omitted from the present study for the following reasons: 3 used frozen nondonor eggs; 2 used donor eggs; 2 did not progress to the embryo transfer stage (1 woman conceived naturally before then); and 4 others were lost to follow-up, leaving a total of 25 patients.
Gynecologic History
All 25 patients in this study had proven or clinically well-supported suspicion of adhesions. Medical diagnoses included:
| Infectious/inflammatory disease | (23) | 92.0% |
| Abdominopelvic surgery | (21) | 84.0% |
| Abdominopelvic trauma | (14) | 56.0% |
| Confirmed pelvic adhesions | (14) | 56.0% |
| Endometriosis | (9) | 36.0% |
| Pelvic inflammatory disease | (2) | 8.0% |
Before treatment, 14/25 patients reported a total of 21 prior natural pregnancies, only 4 of which resulted in a live birth. Twenty patients had a total of 78 prior ART attempts, including 54 IUIs. The total number of prior ART pregnancies was 3; 2 of these ended in miscarriage. Thus, before receiving the therapeutic intervention, there was only 1 prior ART full-term pregnancy in 78 attempts.
Characteristics
The 25 study participants comprised a multiethnic, primarily white, group, ranging in age from 28 to 44 years. At the time of embryo transfer, the mean age was 36 (median, 35.4), and the mean duration of infertility was 4.6 years (median, 3.5).
Procedures/Intervention
A total of 23/25 (92.0%) patients received the recommended minimum of 10 hours of treatment. [Note: As of January 2001, 10 hours was the required minimum.] None of the patients received concurrent infertility therapies during the treatment period.
Data Collection
Study patients were evaluated and treated between September 1998 and January 2003. Approximately 1 year after their last treatment date, patients were contacted to determine whether they had: (1) undergone the embryo transfer phase of IVF therapy, and (2) used fresh nondonor eggs/embryos (vs frozen or donor eggs).
The final data set includes 25 patients who underwent >/=1 IVF transfers within a maximum of 15 months following treatment, using fresh nondonor eggs/embryos. Patients who progressed from embryo transfer to pregnancy were tracked to anticipated delivery date, when possible.
In lieu of asking patients to serve as a control group for this as yet unproven adjunctive therapy, the decision was made to compare the study results with the vast, preexisting control groups represented by the 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports, released by the Centers for Disease Control and Prevention (CDC) and the American Society for Reproductive Medicine (December 2003).[26] The control group data set was extrapolated from the various figures in this report.
By law, the CDC reports its ART success rates by treatment cycles started each year, rather than per patient. In accordance with this convention, women who have started >/= 2 cycles per year are represented more than once. Eight of the 25 women in the present study had 2 cycles each, for a total of 33 cycles. CDC success rates, in all age groups using fresh nondonor eggs/embryos, are somewhat (not significantly) lower for women who underwent a previously unsuccessful ART cycle.[34]
The CDC also reports its National Summary by age groups. Although other factors (eg, infertility cause, number of embryos transferred) should be considered, a woman's age (when using her own eggs) is the primary determinant of success at every stage of the IVF process.[25] Thus, we did not attempt to assess the effects of factors other than age in this study.
Statistical Methods
The main outcome measure—pregnancy/transfer rate (as well as the live birth/transfer rate)—was compared with the CDC 2001 rates (adjusted for age), as follows. For each attempt, the expected rate is the probability of pregnancy and live birth, respectively, for a woman of the identical age in years. CDC report Figure 13 (and its accompanying text)[32] provides these data. There is no material difference in success rates between an unsuccessful first attempt and subsequent ART attempts for the same woman.[34] Because of the small sample sizes, large sample approximations for the Mantel-Haenszel statistic[35] were thought to be unreliable, and 10,000 simulations were used to obtain the 2-sided P value.
Odds ratios were estimated by the following formula, with N = Sample Size, OBS = Observed Total, and EXP = Expected Total based on the CDC 2001 data:
Estimated Odds Ratio = OBS(N-EXP)/[(N-OBS)EXP]
A 95% confidence interval for the odds ratio was obtained via 10,000 simulations, finding the odds ratios that make the P value .025 and .975.
Results
To assess the effectiveness of this site-specific manual soft-tissue therapy in improving pregnancy rates in women undergoing subsequent IVF, the main outcome measure was clinical pregnancy via the transfer of fresh embryos from nondonor eggs, within 15 months of the last (manual) treatment date.
As discussed above, this study included only those patients who had progressed beyond the early stages (egg production/retrieval and fertilization) of the ART cycle to the embryo transfer stage, and had used fresh nondonor eggs/embryos. Accordingly, the study results are compared with the 81% (n = 65,353) of the CDC cycles that reached the embryo transfer stage. These numbers were extrapolated from various figures in the 2001 report.[27,29,32,33]
As shown in Table 4, for pregnancies, based on 33 transfers, there were 22 successes. The CDC age-adjusted expected rate was 12.7 successes; and the standard error in the observed rate is 2.7 (P < .001).
Table 4.
Observed vs Expected Pregnancies
| Age | Transfers | Pregnancies | ||
| Observed | CDC 2001 Rate | Expected | ||
| < 35 | 15 | 11 (73%) | 47.7% | 7.15 |
| 35–37 | 6 | 4 (67%) | 42.0% | 2.52 |
| 38–40 | 5 | 3 (60%) | 33.8% | 1.69 |
| 41–42 | 4 | 3 (75%) | 23.6% | 0.94 |
| <42 | 3 | 1 (33%) | 12.1% | 0.36 |
| Total | 33 | 22 | 12.7 | |
Observed = rate for Manual pre-IVF treatment (study)
Expected = projected from CDC Figure 13[32]
(Expected = Transfers times published CDC 2001 probability. For example, the published CDC 2001 pregnancy rate for a <35 year old woman is 47.7%, hence with 15 transfers, one expects 15 x 0.477 = 7.15 pregnancies; the observed number was 11.)
The estimated age-standardized pregnancy odds ratio of manual treatment pre-IVF to no pretreatment is 3.20 (95% confidence interval 1.55–8.4). As an example, if the odds of success for a control treatment is 1:2 vs the odds of success for an experimental treatment of 2:1, the odds ratio is 2.0/0.5 = 4.0. Note that equivalence corresponds to an odds ratio of 1.00, which is excluded from the pregnancy interval but not from the live birth interval (below).
The CDC pregnancy rates per age group ranged from 12% (age > 42) to 48% (age < 35). In comparison, the pre-IVF study pregnancy rates ranged from a low of 33% (age > 42) to more than 70% (age < 35).
Speaking in terms of actual patients, rather than embryo transfers, clinical pregnancies were documented in 19 of 25 women. The mean number of treatment hours was 17.1. There was no meaningful difference in treatment time between those who progressed from transfer to pregnancy (mean, 16.9 hours) and those who did not (mean, 17.5 hours).
Although the main outcome measure of this study was pregnancy within 15 months of the last (manual) treatment date, 15 of 33 transfers have resulted in live births or continuing pregnancies. As seen in Table 5, the CDC age-adjusted expected number was 10.3, and the standard error for the observed rate was 2.6 (P = .065). Similarly, it can be estimated that the age-standardized successful live birth odds ratio of manual treatment pre-IVF to no treatment is 1.86 (95% confidence interval 0.86–4.3).
Table 5.
Observed vs Expected Live Births
| Age | Transfers | Pregnancies | ||
| Observed | CDC 2001 Rate | Expected | ||
| < 35 | 15 | 9 (60%) | 41.1% | 6.16 |
| 35–37 | 6 | 2 (33%) | 35.1% | 2.11 |
| 38–40 | 5 | 1 (20%) | 25.4% | 1.27 |
| 41–42 | 4 | 2 (20%) | 14.5% | 0.58 |
| >42 | 3 | 1 (33%) | 6.1% | 0.18 |
| Total | 33 | 15 | 10.3 | |
Observed = rate for Manual pre-IVF treatment (study)
Expected = projected from CDC National Summary, Figure 13.[29, 32]
(Expected = Transfers times published CDC 2001 probability. For example, the published CDC 2001 live birth rate for a <35 year old woman is 41.1%, hence with 15 transfers, one expects 15 x 0.411 = 6.16 live births; the observed number was 9.)
The confidence interval indicates that plausible outcomes range from a clinically insignificant disadvantage to a clinically important advantage for this pre-IVF treatment over common medical practice in terms of live births. Again, speaking in terms of actual patients, rather than embryo transfers, 15/25 women have already delivered (n = 13) or are still pregnant (n = 2).
Treatment Safety.
None of the patients in the study reported any observable complications or adverse side effects as a result of their treatment; and all but 1 patient who presented with pain at the initial evaluation reported decreased pain during or after treatment.
Discussion
Although we can infer that the entire confidence interval for the pregnancy odds ratio is clinically significant, the confidence interval for live births contains both clinically insignificant values (eg, near 1.0), as well as clinically significant values. However, the confidence interval for the odds ratio demonstrates the potential for anything from a slightly lower rate to a much higher rate. As can be seen in Table 4 and Table 5, the results were particularly encouraging for women > 40 years of age. Thus, further research with a larger sample is needed to define the successful live birth confidence interval more precisely.
Nevertheless, because national live births/transfer rates have been steadily improving (from 28.0% in 1996 to 33.4% in 2001),[33] an intervention that obviously increases clinical pregnancy rates should increase live birth delivery rates for patients undergoing IVF embryo transfers.
Related Research
In terms of the efficacy of alternative therapies as pre-IVF aids, there is 1 published, randomized controlled trial of the effect of acupuncture on the pregnancy rate of women undergoing IVF or intracytoplasmic sperm injection. The 160 patients (mean age, 32.5) in this German study were randomly assigned to the acupuncture or control group. The main outcome measure was clinical pregnancy. Analysis showed that the average pregnancy rate for the acupuncture group was 42.5% (34/80) vs 26.3% (21/80) for the control group (P = .03).[36] Investigators have concluded that further studies are warranted.[37,38] The acupuncture results can be compared with the average 66.7% (22/33) pregnancy rate obtained in the present study, which used the [much higher] 2001 CDC average pregnancy/transfer rate of 40.6% as the control group (P < .001). [Also see Future research, below.]
Future Research
As with Study I, the encouraging results warrant the replication of Study II, using a considerably larger sample of women (particularly in the age 35+ groups) randomized into experimental (treatment) and control (no treatment) groups. A second control group, composed of infertile women lacking strong indications of adhesion formation, would permit testing the hypothesis that this specific therapy might also benefit infertile women without adhesions, eg, by improving circulation in the pelvic region. The results of this arm of the study could be compared with acupuncture, which seems to work (in part) by increasing blood flow to the uterus.[37]
Conclusion
The data trend across the pilot and present studies seems to support the hypothesis that this distinctive protocol of site-specific manual soft-tissue therapy facilitates fertility in women with a wide array of adhesion-related biomechanical dysfunctions. The major indication for its use is a history suggesting abdominopelvic adhesions (ie, prior surgery, infection, inflammation, or trauma at the reproductive organs or neighboring structures).
This innovative, noninvasive, nonsurgical, manual therapeutic technique confers little risk and few adverse side effects or complications, and appears to be an effective treatment for facilitating natural fertility and improving pregnancy rates/embryo transfer in women undergoing subsequent IVF. Thus, it can be prescribed as an alternative or complementary treatment to standard gynecologic care and should be considered as a new adjunct to existing medical infertility treatments.*
Acknowledgments
We would like to thank Thom L. Tyler, MD, PhD, Gainesville, FL and Michael Davidson, DC (UK) for encouraging us in this endeavor. We also thank Gerald Wiechmann, PhD (former Sr. Health Research Advisor, NIH); Cynthia Hodgson, PT, PhD; Sandra Shevlin, DP; and Kimberley Hornberger, PTA for research, writing, and editorial assistance. Lastly, we acknowledge the crucial contributions of Amy B. Hough, our meticulous research assistant.
Note
*As this therapy was directed toward (female) mechanical infertility and would have no effect on male factor nor any anticipated effects on female medical/hormonal infertility (ie, diminished ovarian reserve, ovulatory dysfunction), the patients in this study were limited to those who reached the embryo transfer stage of the ART cycle. That is, they had progressed beyond the egg production, egg retrieval, and fertilization steps. Fresh, nondonor eggs/embryos were preferred not only by our patients but are used in approximately 75% of all ART cycles.[28]
Footnotes
*Wurn Technique®, patent pending
Contributor Information
C. Richard King, Florida Medical and Research Institute, P.A., Gainesville, Florida.
Marvin A Heuer, College of Medicine, University of Florida, Gainesville, and Iovate Health Sciences Services, Inc. Toronto, Ontario.
Eugenia S Scharf, medical writer/researcher, Gainesville, Florida.
Jonathan J Shuster, Dept. of Statistics, College of Medicine, University of Florida, Gainesville.
References
- 1. Mosher W, Pratt W. Fecundity and infertility in the United States, 1965-88. Advance Data, National Center for Health Statistics, Centers for Disease Control and Prevention, USDHHS/PHS, no. 192, December 4, 1990:2-6.
- 2. Meldrum DR. Infertility. In: Hacker NF, Moore JG. Essentials of Obstetrics and Gynecology. Philadelphia, Pa: W.B. Saunders; 1992:444. [Google Scholar]
- 3. World Health Organization. Infertility: a tabulation of available data on prevalence of primary and secondary infertility. Geneva, Switzerland: WHO Programme on Maternal and Child Health and Family Planning; 1991. [Google Scholar]
- 4. Strickler RC. Factors influencing fertility. In: Keye WR, Chang RJ, Rebar RW, Soules MR, eds. Infertility: Evaluation and Treatment. Philadelphia, Pa: W.B. Saunders; 1995:8-18. [Google Scholar]
- 5. Stephen EH. Projections of impaired fecundity among women in the United States: 1995 to 2020. Fertil Steril. 1996; 66: 205-209. [DOI] [PubMed] [Google Scholar]
- 6. Drollette CM, Badawy SZ. Pathophysiology of pelvic adhesions. Modern trends in preventing infertility. J Reprod Med. 1992; 37: 107-122. [PubMed] [Google Scholar]
- 7. Stone K. Adhesions in gynecologic surgery. Curr Opin Obstet Gynecol. Curr Opin Obstet Gynecol. 1993; 5: 322-327. [PubMed] [Google Scholar]
- 8. Steege JF, Stout AL. Resolution of chronic pelvic pain after laparoscopic lysis of adhesions. Am J Gynecol. 1991; 165: 278-283. [DOI] [PubMed] [Google Scholar]
- 9. Diamond MP, Copperman AB. Treatment of disorders of the fallopian tube. In: Keye WR, Chang RJ, Rebar RW, Soules MR, eds. Infertility: Evaluation and Treatment. Philadelphia, Pa: W.B. Saunders; 1995:474-482. [Google Scholar]
- 10. Bahamondes L, Bueno JG, Hardy E, et al. Identification of main risk factors for tubal infertility. Fertil Steril. 1994; 6: 478-482. [PubMed] [Google Scholar]
- 11. Silber S. How to Get Pregnant With the New Technology. New York, NY: Warner Books; 1991:42,102-109, 170, 202. [Google Scholar]
- 12. Threlkeld AJ. The effects of manual therapy on connective tissue. Phys Ther. 1992; 72: 893-902. [DOI] [PubMed] [Google Scholar]
- 13. Maitland GD. Vertebral Manipulation, 5th ed. London, UK: Butterworth & Co.; 1986:3-4. [Google Scholar]
- 14. Ellis H. The cause and prevention of postoperative intraperitoneal adhesions. Surg Gynecol Obstet. 1971; 133: 497-511. [PubMed] [Google Scholar]
- 15. Raftery AT. Effect of peritoneal trauma on peritoneal fibrinolytic activity and intraperitoneal adhesion formation. Eur Surg Res. 1981; 13: 397-401. [DOI] [PubMed] [Google Scholar]
- 16. Holtz G. Prevention and management of peritoneal adhesions. Fertil Steril. 1984; 41: 497-507. [DOI] [PubMed] [Google Scholar]
- 17. Cummings GS, Crutchfield CA, Barnes MR. Orthopedic physical therapy series: Soft Tissue Changes in Contractures. Atlanta, Ga: Stokesville; 1983:1. [Google Scholar]
- 18. Kresch AJ, Seifer DB, Sachs LB, Barrese I. Laparoscopy in 100 women with chronic pelvic pain. Obstet Gynecol. 1984; 64: 672-674. [PubMed] [Google Scholar]
- 19. Diamond MP, Hershlag A. Adhesion formation/reformation. Prog Clin Biol Res. 1990; 358: 22-33. [PubMed] [Google Scholar]
- 20. Holmdahl L. The role of fibrinolysis in adhesion formation. Eur J Surg Suppl. 1997; 577: 24-31. [PubMed] [Google Scholar]
- 21. Mojzis L, Nemec R, Hlavaty V. Children of Your Own: the Mojzis Method. Boulder, Colo: Richmond Bay; 1990. [Google Scholar]
- 22. Volejnikova H. Female infertility: a study of physical treatment by the method of L. Mojzisovà for functional disturbances of the pelvic region. J Orthopaedic Med. 2001; 23: 47-49. [Google Scholar]
- 23. Wiechmann G, Wurn B, Wurn L. Manual Soft Tissue Therapy to Decrease Abdominopelvic Adhesions. Gainesville, Fla: Clear Passage Therapies; 1997. [Google Scholar]
- 24. Wiechmann G, Wurn B, Wurn L. Manual Soft Tissue Therapy to Decrease Abdominopelvic Adhesions. Gainesville, Fla: Clear Passage Therapies; 1997. [Google Scholar]
- 25. Davis OK, Rosenwaks Z. In Vitro Fertilization. In: Keye WR, Chang RJ, Rebar RW, Soules MR, eds. Infertility: Evaluation and Treatment. Philadelphia, Pa: W.B. Saunders; 1995: 759-771. [Google Scholar]
- 26. 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, Ga: Centers for Disease Control and Prevention and the American Society for Reproductive Medicine, 2003: [Fig. 40, p 52.] [Google Scholar]
- 27. Ibid, Fig. 3, p 15. [Google Scholar]
- 28. Ibid, Fig. 3, p 15. [Google Scholar]
- 29. Ibid, National Summary, p 71. [Google Scholar]
- 30. Ibid, National Summary, p 71. [Google Scholar]
- 31. Ibid, Glossary, p 467. [Google Scholar]
- 32. Ibid, Fig. 13, p 25. [Google Scholar]
- 33. Ibid, Fig. 41, p 53. [Google Scholar]
- 34. Ibid, Fig. 20, p 32. [Google Scholar]
- 35. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22: 719-748. [PubMed] [Google Scholar]
- 36. Paulus WE, Zhang M, Strehler E, El-Danasouri I, Sterzik K. Influence of acupuncture on the pregnancy rate in patients who undergo assisted reproduction therapy. Fertil Steril. 2002; 77: 721-724. [DOI] [PubMed] [Google Scholar]
- 37. Chang R, Chung PH, Rosenwaks Z. Role of acupuncture in the treatment of female infertility. Fertil Steril. 2002; 78: 1149-1153. [DOI] [PubMed] [Google Scholar]
- 38. White AR. A review of controlled trials of acupuncture for women's reproductive health care. J Fam Plann Reprod Health Care. 2003; 29: 233-236. [DOI] [PubMed] [Google Scholar]


