Abstract
1. Pituitary glands of adult rats of both sexes, of lactating female and of new-born rats, incubated in a Locke solution, release both oxytocin and vasopressin. The amount of hormones released, during a measured period of incubation, is related to the actual hormone content of the gland.
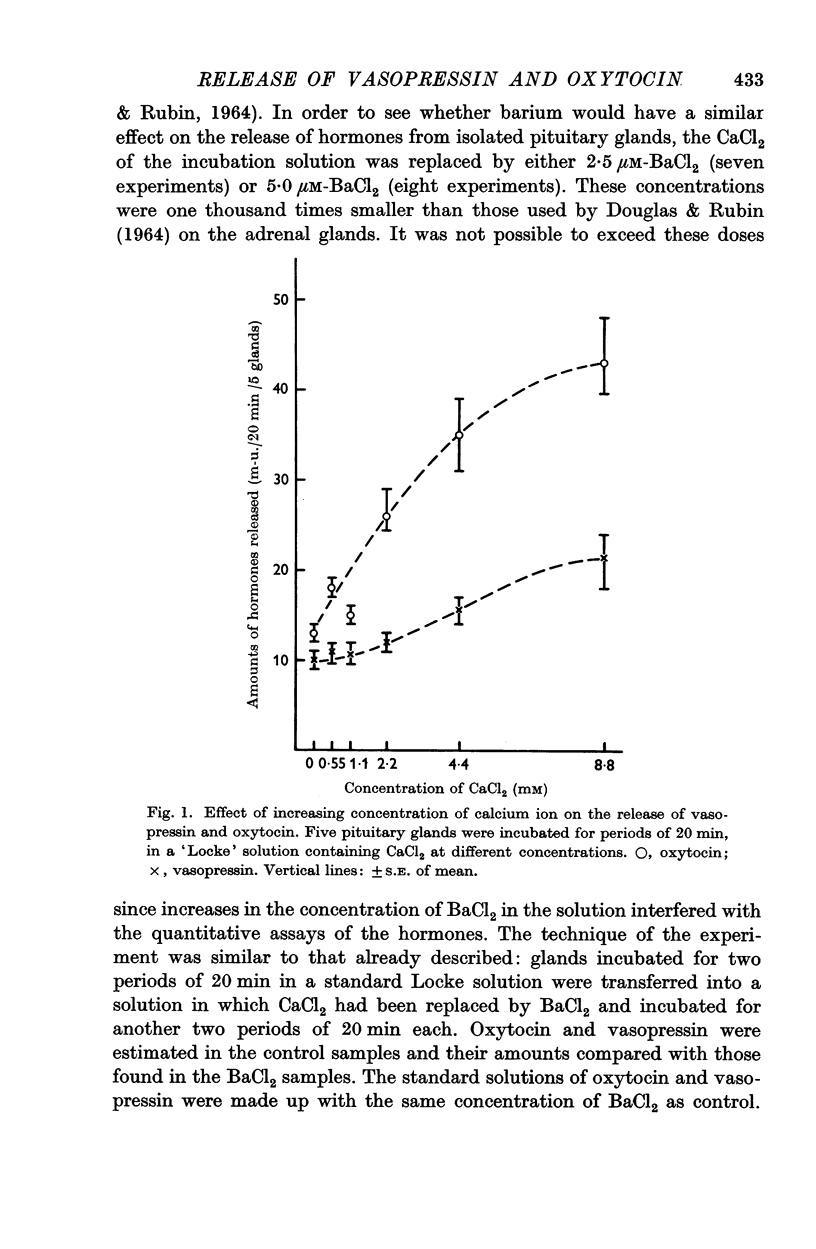
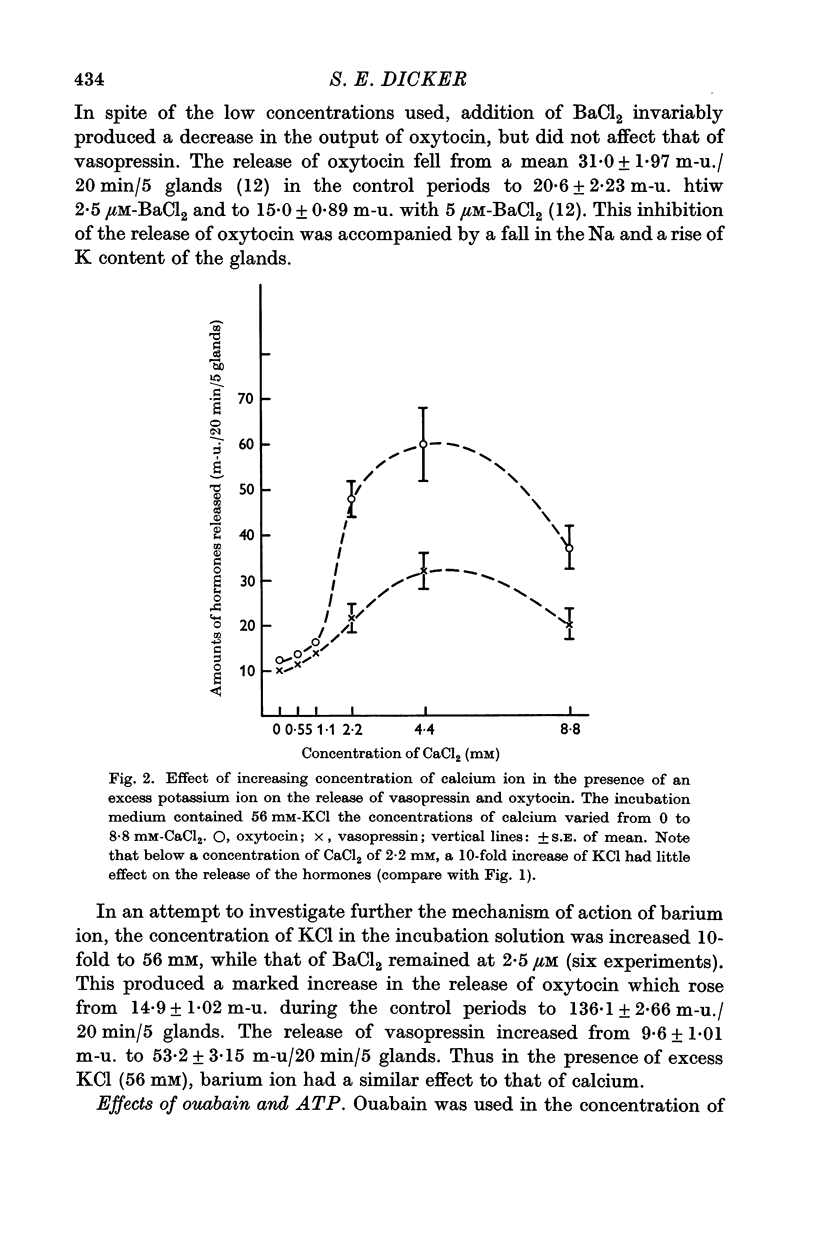
2. Increasing the concentration of KCl in the incubation medium, with CaCl2 present and in concentration of at least 2·2 mM, produces an enhanced release of both hormones from pituitary glands of adults, but does not affect the release of hormones from glands of new-born animals.
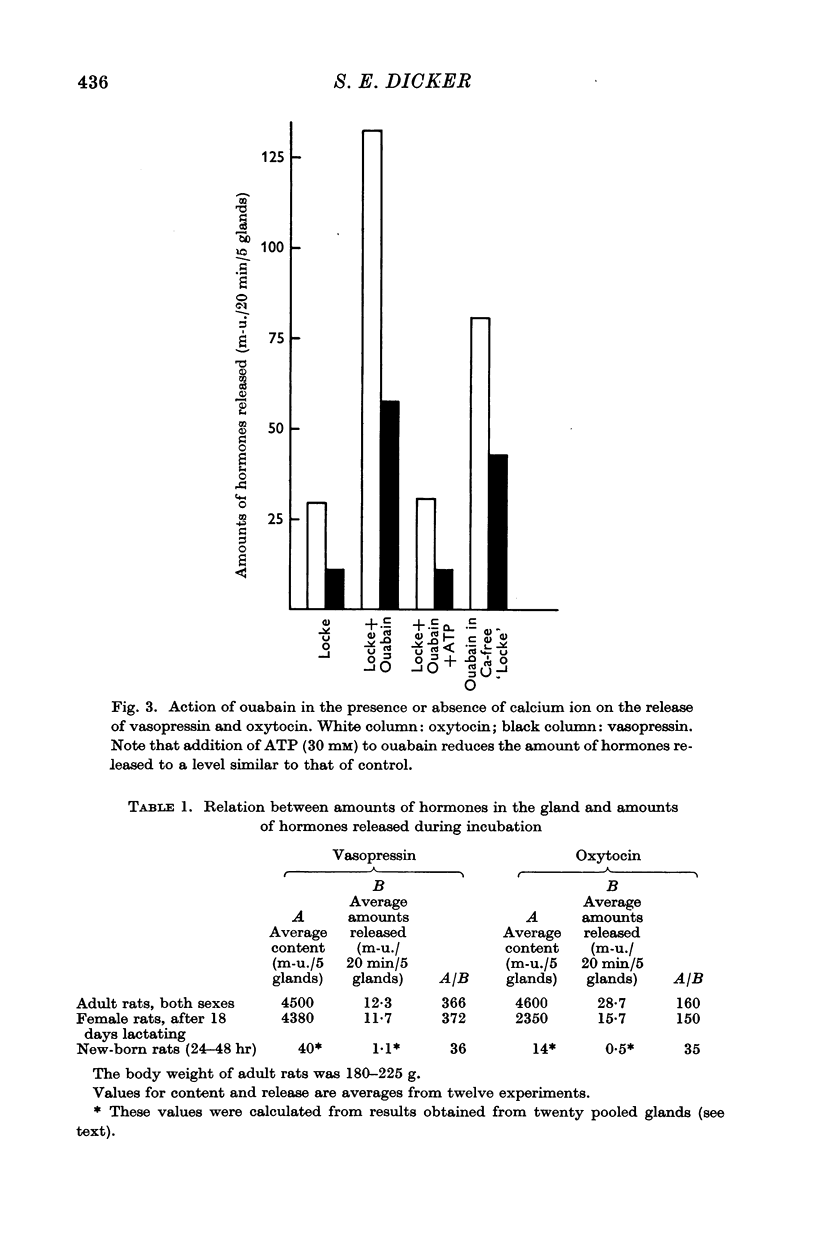
3. Addition of ouabain to the incubation medium produces a marked increase of the release of the hormones from glands of both adult and new-born rats. This is accompanied by an extrusion of K ion and an influx of Na ion. The effect of ouabain on the hormone release and the shift of ions can be reversed by subsequent addition of adenosine triphosphate.
4. The increased release of hormones produced by ouabain, in glands from new-born rats, is unaffected by the presence or absence of CaCl2. In adults, however, the effect of ouabain, though present, is reduced in the absence of CaCl2.
5. It is suggested that in glands from adult animals, the hormones must be freed from their attachment on the protein-carrier, neurophysin and that this can be achieved by the entry of calcium ion into the cell. The subsequent secretion of the `freed' hormones appears to be accompanied by a shift of ions across the cell membrane.
6. In glands from neonates up to 3 weeks old, the absence of neurophysin, or its poor capacity for binding the hormones, explains the inability of calcium to operate in the same way as in the glands of adults. There is evidence suggesting that the secretion of the neurohypophysial hormones in the new-born animal consists mainly of their diffusion from the cells, without previous elution of the hormones as in adults.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CHAUVET J., OLIVRY G. Sur l'existence éventuelle d'une hormone unique neurohypophysaire. I. Relations entre l'ocytocine, la vasopressine et la protéine de Van Dyke extraites de la neurohypophyse du boeuf. Biochim Biophys Acta. 1956 Dec;22(3):421–427. doi: 10.1016/0006-3002(56)90050-6. [DOI] [PubMed] [Google Scholar]
- BARER R., HELLER H., LEDERIS K. THE ISOLATION, IDENTIFICATION AND PROPERTIES OF THE HORMONAL GRANULES OF THE NEUROHYPOPHYSIS. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:388–416. doi: 10.1098/rspb.1963.0054. [DOI] [PubMed] [Google Scholar]
- BARGMANN W. Ueber die neurosekretorische Verknüpfung von Hypothalamus und Neurophypophyse. Z Zellforsch Mikrosk Anat. 1949;34(5):610–634. [PubMed] [Google Scholar]
- BELL E. T., LORAINE J. A., MUKERJI S., VISUTAKUL P. FURTHER OBSERVATIONS ON THE OVARIAN ASCORBIC ACID DEPLETION TESTS FOR LUTEINIZING HORMONE. J Endocrinol. 1965 Apr;32:1–7. doi: 10.1677/joe.0.0320001. [DOI] [PubMed] [Google Scholar]
- BENIRSCHKE K., MCKAY D. G. The antidiuretic hormone in fetus and infant; histochemical observations with special reference to amniotic fluid formation. Obstet Gynecol. 1953 Jun;1(6):638–649. [PubMed] [Google Scholar]
- CAESAR R., RODECK H. Zur Entwicklung des neurosekretorischen Systems bei Säugern und Mensch und der Regulationsmechanismen des Wasserhaushaltes. Z Zellforsch Mikrosk Anat. 1956;44(6):666–691. [PubMed] [Google Scholar]
- CROSS B. A., HARRIS G. W. The role of the neurohypophysis in the milk-ejection reflex. J Endocrinol. 1952 Apr;8(2):148–161. doi: 10.1677/joe.0.0080148. [DOI] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., EGGLETON M. G. Hyaluronidase and antidiuretic activity in urine of man. J Physiol. 1960 Dec;154:378–384. doi: 10.1113/jphysiol.1960.sp006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., TYLER C. Estimation of the antidiuretic, vasopressor and oxytocic hormones in the pituitary gland of dogs and puppies. J Physiol. 1953 Apr 28;120(1-2):141–145. doi: 10.1113/jphysiol.1953.sp004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., TYLER C. Vasopressor and oxytocic activities of the pituitary glands of rats, guinea-pigs and cats and of human foetuses. J Physiol. 1953 Jul;121(1):206–214. doi: 10.1113/jphysiol.1953.sp004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. THE PREPARATION OF BOVINE NEUROPHYSIN AND THE ESTIMATION OF ITS MAXIMUM CAPACITY TO BIND OXYTOCIN AND ARGININE VASOPRESSIN. J Endocrinol. 1965 May;32:187–198. doi: 10.1677/joe.0.0320187. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., VAN BREEMEN V. L. Electron microscopy of the pituitary and observations on neurosecretion. Am J Anat. 1955 Sep;97(2):177–227. doi: 10.1002/aja.1000970202. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Uncoupling the sodium pump. Nature. 1965 Sep 4;207(5001):1098–1099. doi: 10.1038/2071098a0. [DOI] [PubMed] [Google Scholar]
- HELLER H., LEDERIS K. Maturation of the hypothalamoneurohypophysial system. J Physiol. 1959 Sep 2;147:299–314. doi: 10.1113/jphysiol.1959.sp006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H. The renal function of newborn infants. J Physiol. 1944 Apr 4;102(4):429–440. doi: 10.1113/jphysiol.1944.sp004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABELLA F. S., BEAULIEU G., REIFFENSTEIN R. J. Evidence for the existence of separate vasopressin and oxytocin containing granules in the neurohypophysis. Nature. 1962 Jan 13;193:173–174. doi: 10.1038/193173a0. [DOI] [PubMed] [Google Scholar]
- Opit L. J., Charnock J. S. A molecular model for a sodium pump. Nature. 1965 Oct 30;208(5009):471–474. doi: 10.1038/208471a0. [DOI] [PubMed] [Google Scholar]
- PARDOE A. U., WEATHERALL M. The intracellular localization of oxytocic and vasopressor substances in the pituitary glands of rats. J Physiol. 1955 Jan 28;127(1):201–212. doi: 10.1113/jphysiol.1955.sp005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAMOS M. H., LAVINE L. S., SHAMOS M. I. Piezoelectric effect in bone. Nature. 1963 Jan 5;197:81–81. doi: 10.1038/197081a0. [DOI] [PubMed] [Google Scholar]
- SMITH M. W., THORN N. A. THE EFFECTS OF CALCIUM ON PROTEIN-BINDING AND METABOLISM OF ARGININE VASOPRESSIN IN RATS. J Endocrinol. 1965 May;32:141–151. doi: 10.1677/joe.0.0320141. [DOI] [PubMed] [Google Scholar]
- Thorn N. A. Role of calcium in the release of vasopressin and oxytocin from posterior pituitary protein. Acta Endocrinol (Copenh) 1965 Nov;50(3):357–364. doi: 10.1530/acta.0.0500357. [DOI] [PubMed] [Google Scholar]


