Abstract
1. L-Noradrenaline (NA), 5-hydroxytryptamine (5-HT) and acetylcholine (ACh) were administered micro-electrophoretically to feline lumbar neurones while recording their spike potentials extracellularly.
2. There was no evidence to suggest that NA acts as an excitatory transmitter in the spinal cord.
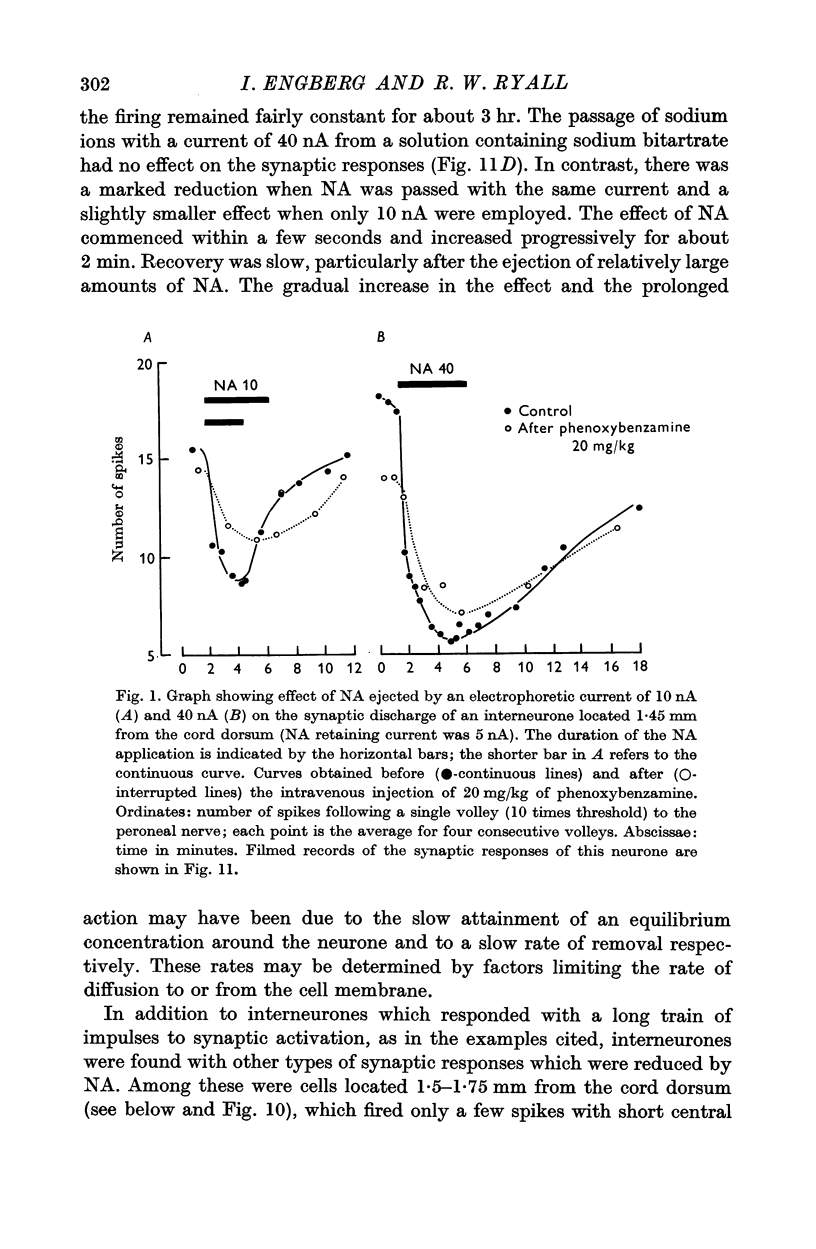
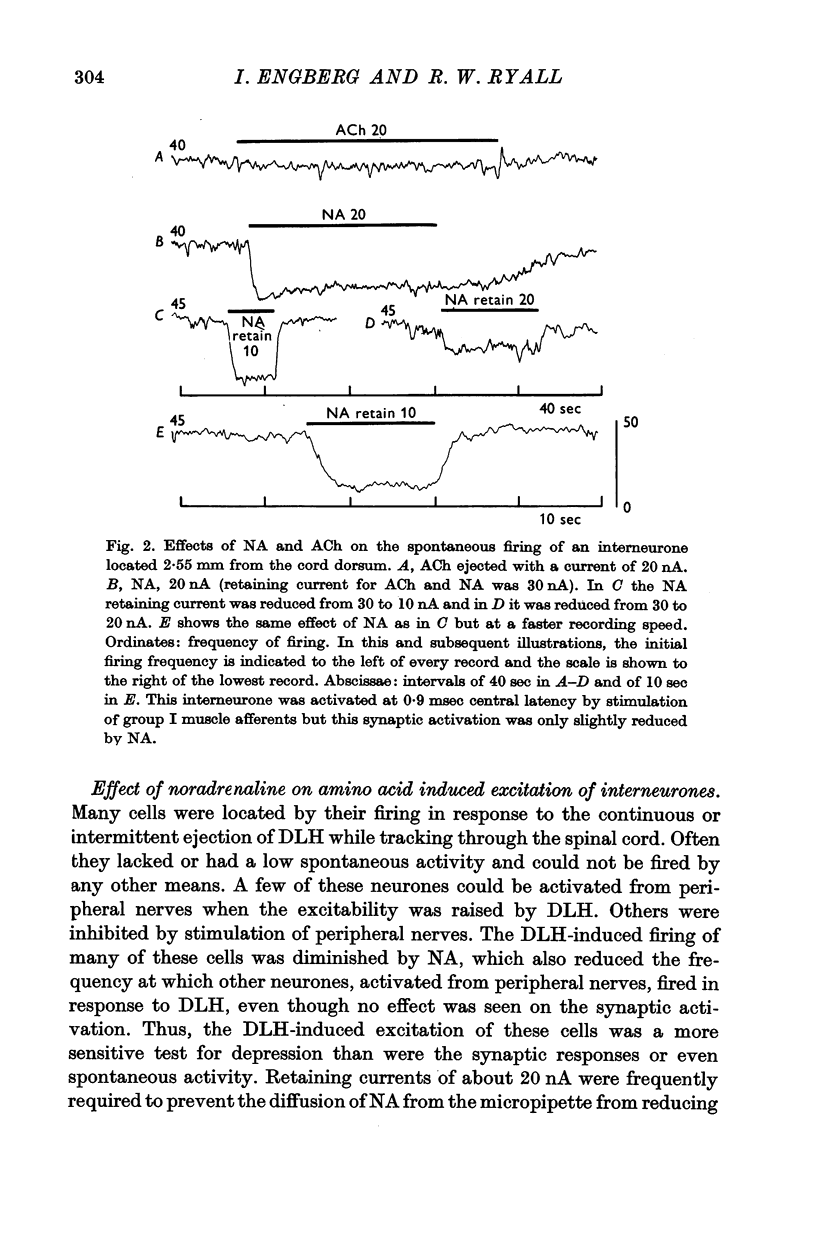
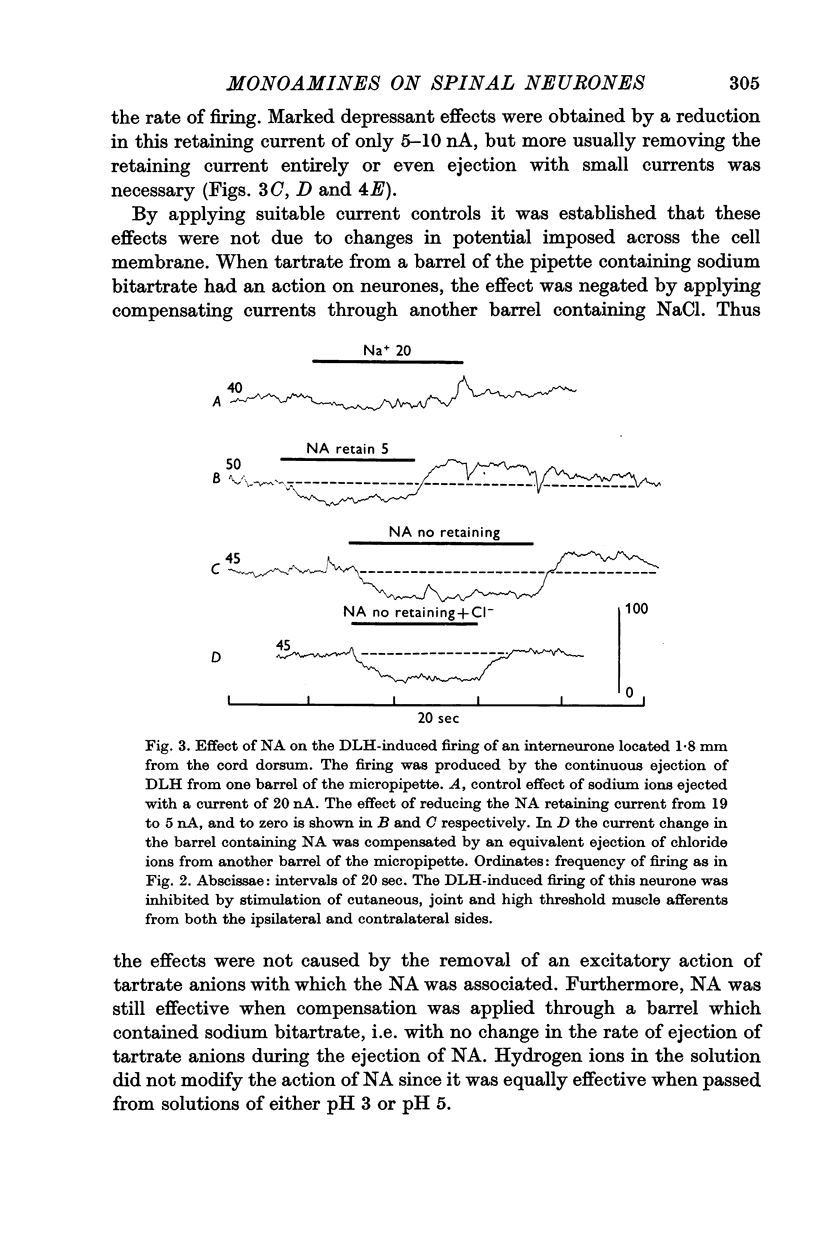
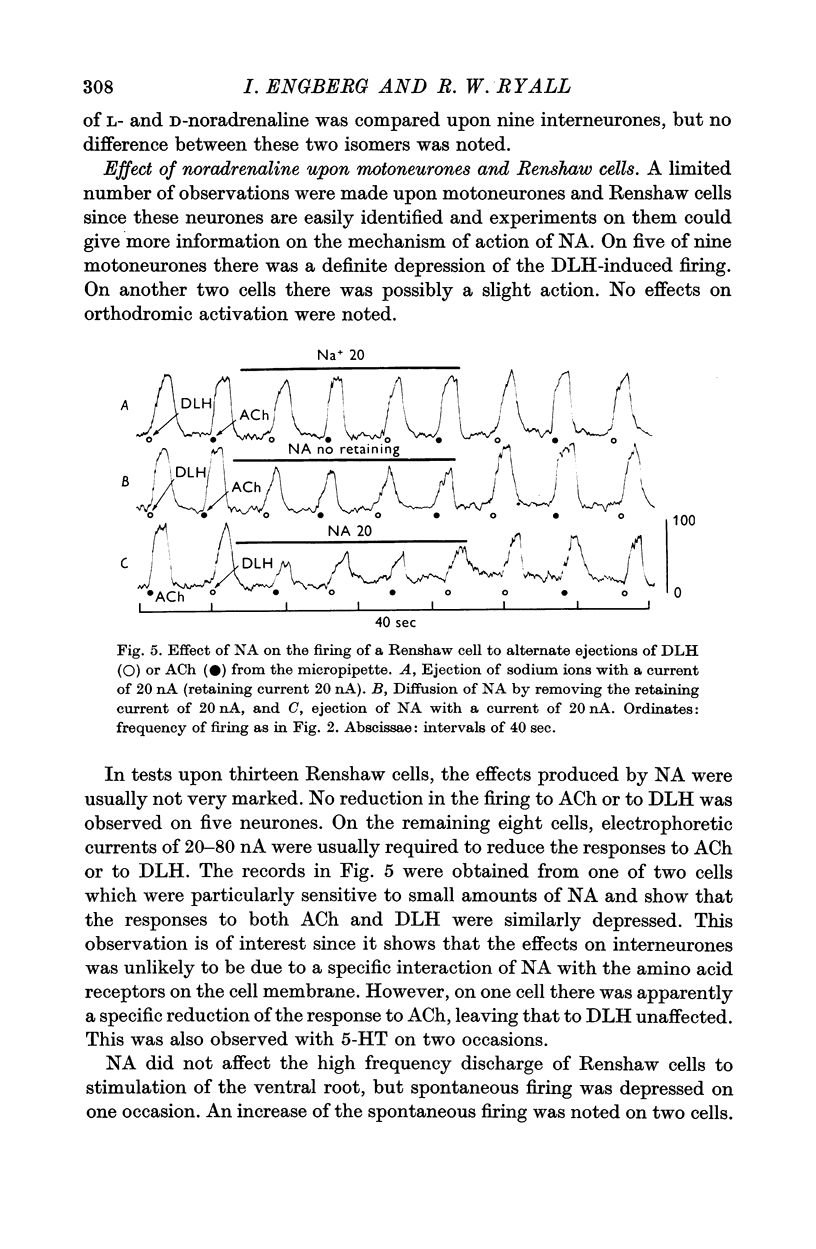
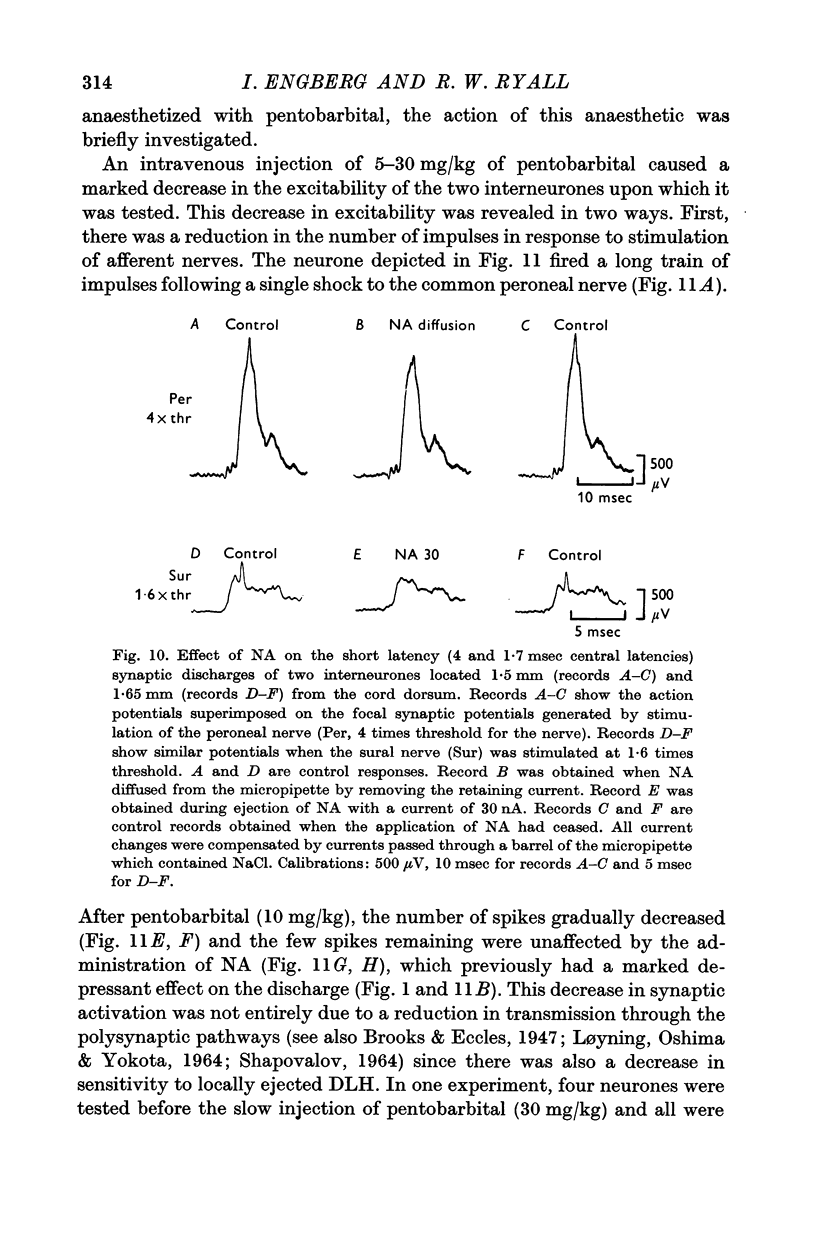
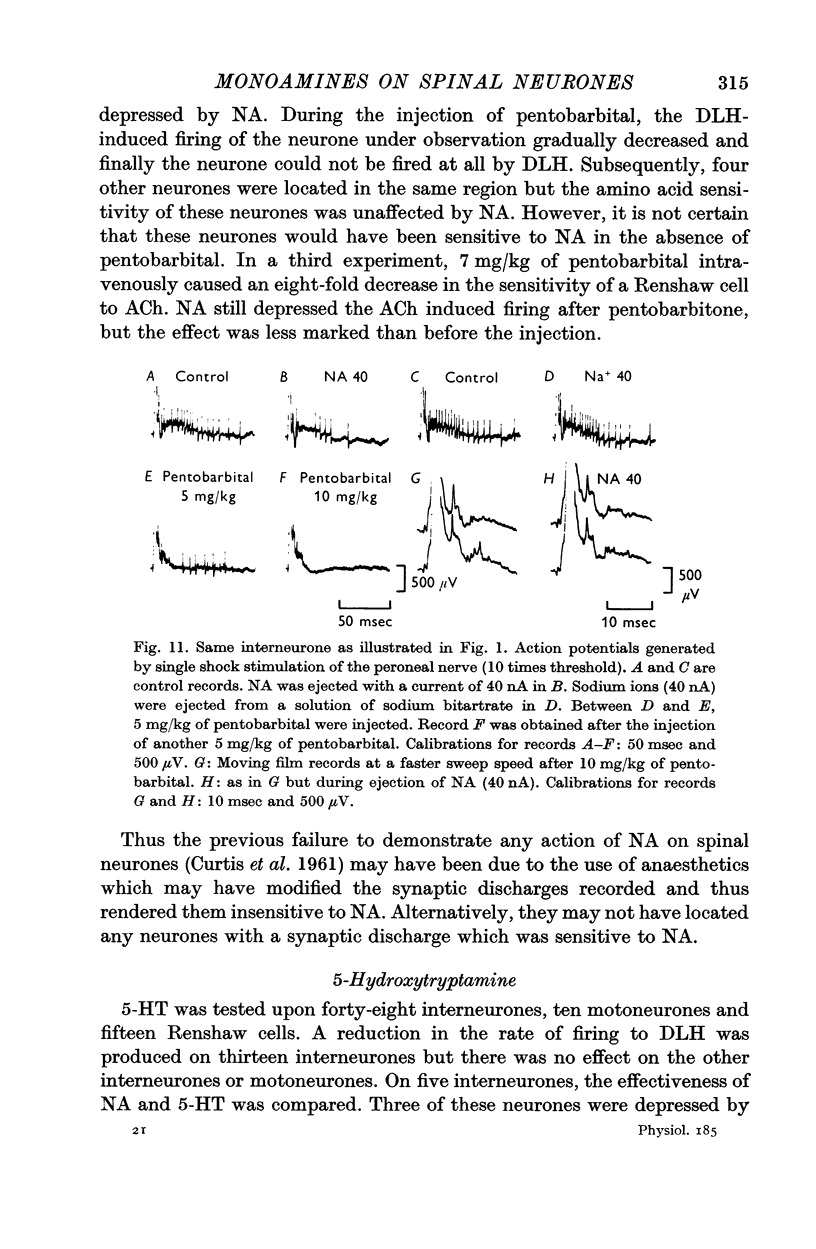
3. NA had potent inhibitory effects on some interneurones as revealed by a depression of spontaneous and synaptic firing and on the firing to a local application of an excitant amino acid. The effects on Renshaw cells and motoneurones were less marked.
4. The depressant actions of 5-HT were less marked than those of NA. ACh and carbamylcholine had depressant effects on some NA-sensitive interneurones but were invariably far less potent and on other NA-sensitive cells were completely inactive.
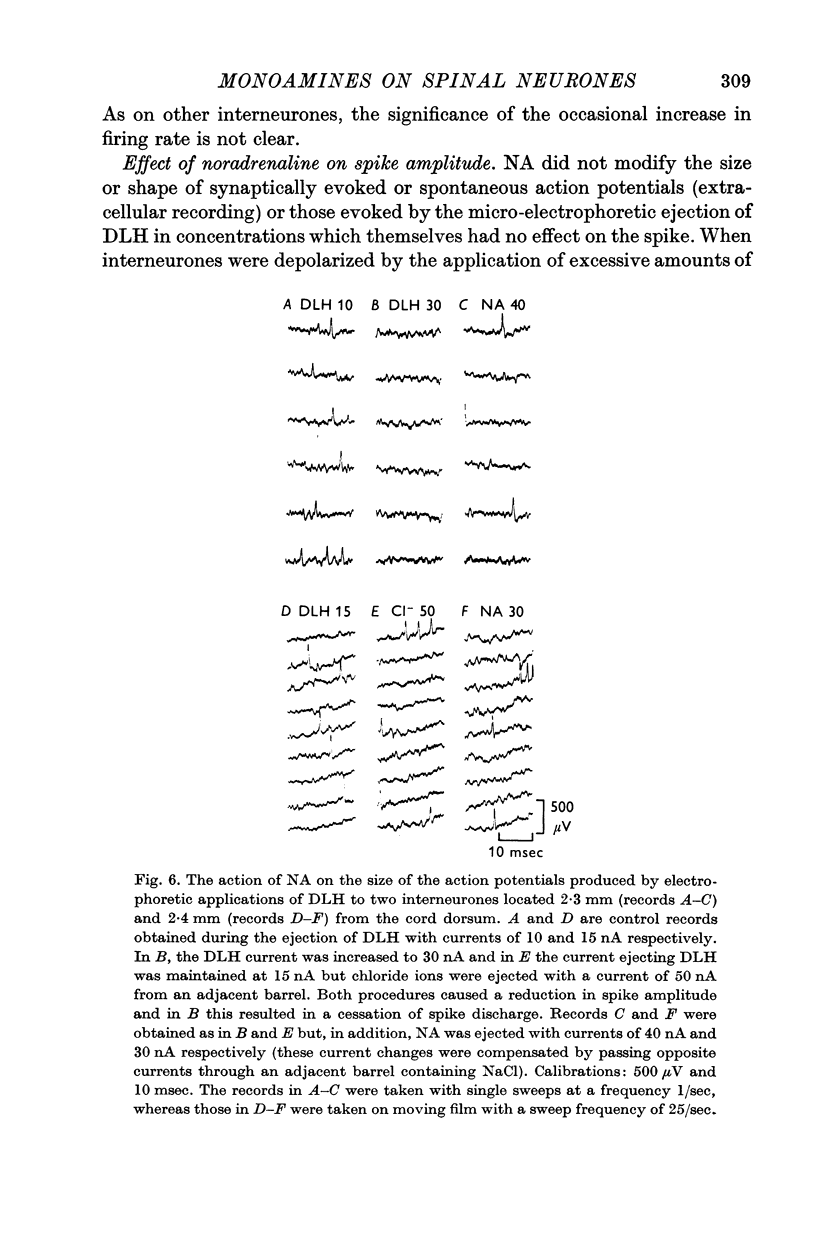
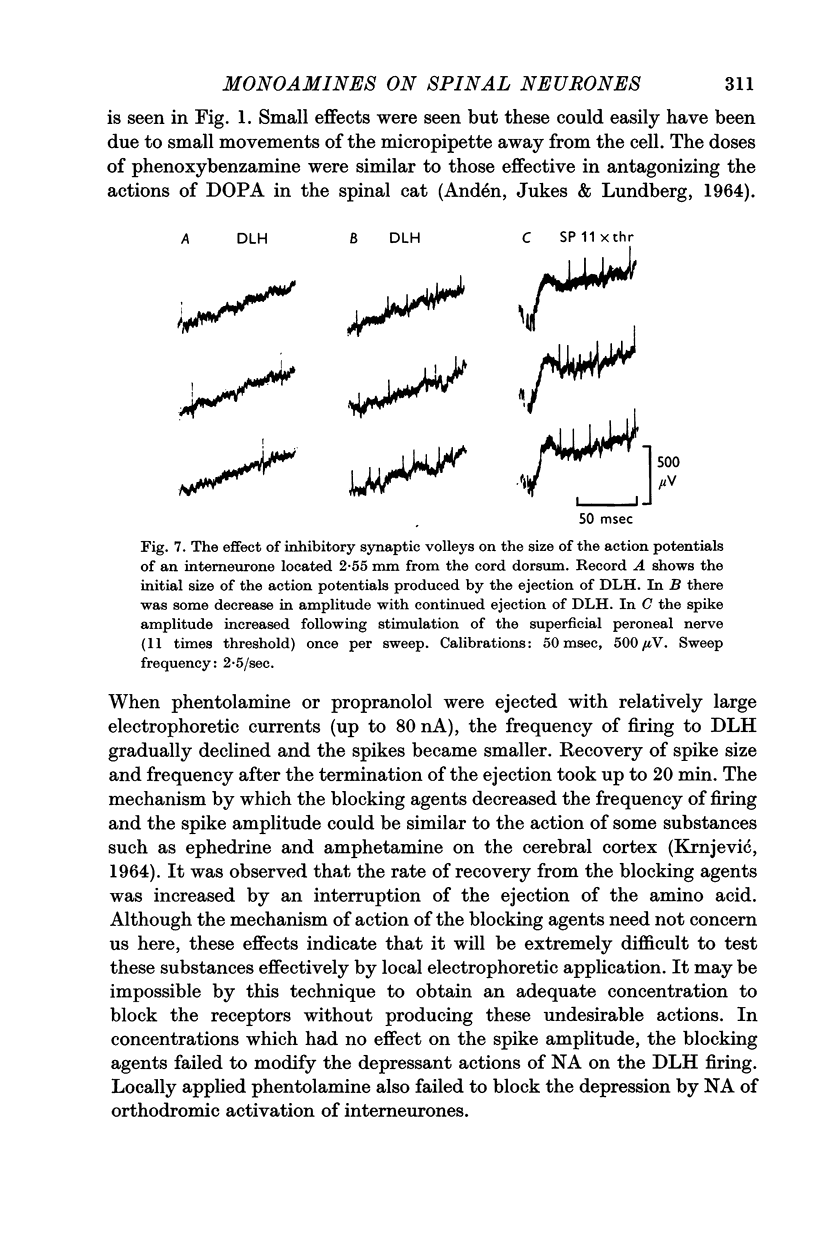
5. NA had no detectable effect on the normal spike amplitude but when the action potentials were reduced by excessive depolarization then both NA and synaptic inhibition increased the spike amplitude; this effect could be due to a hyperpolarization of the cell membrane.
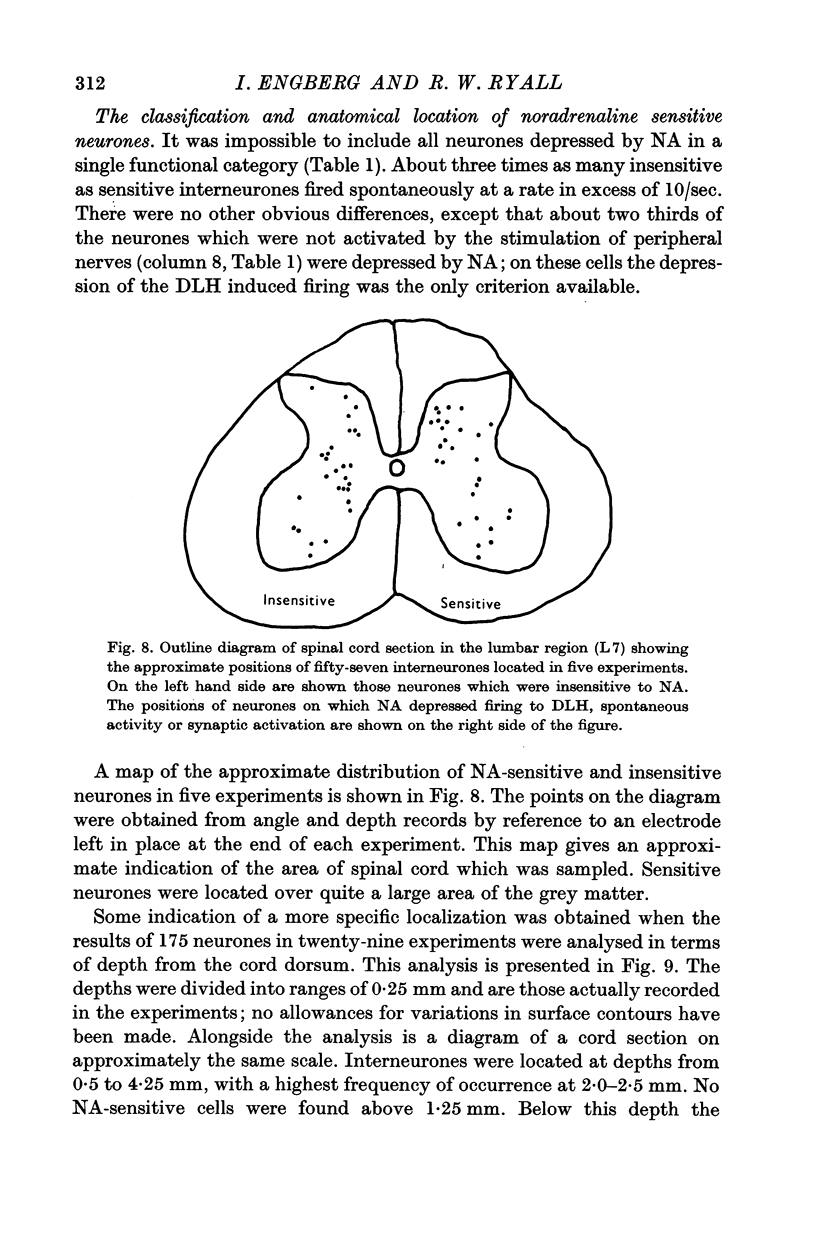
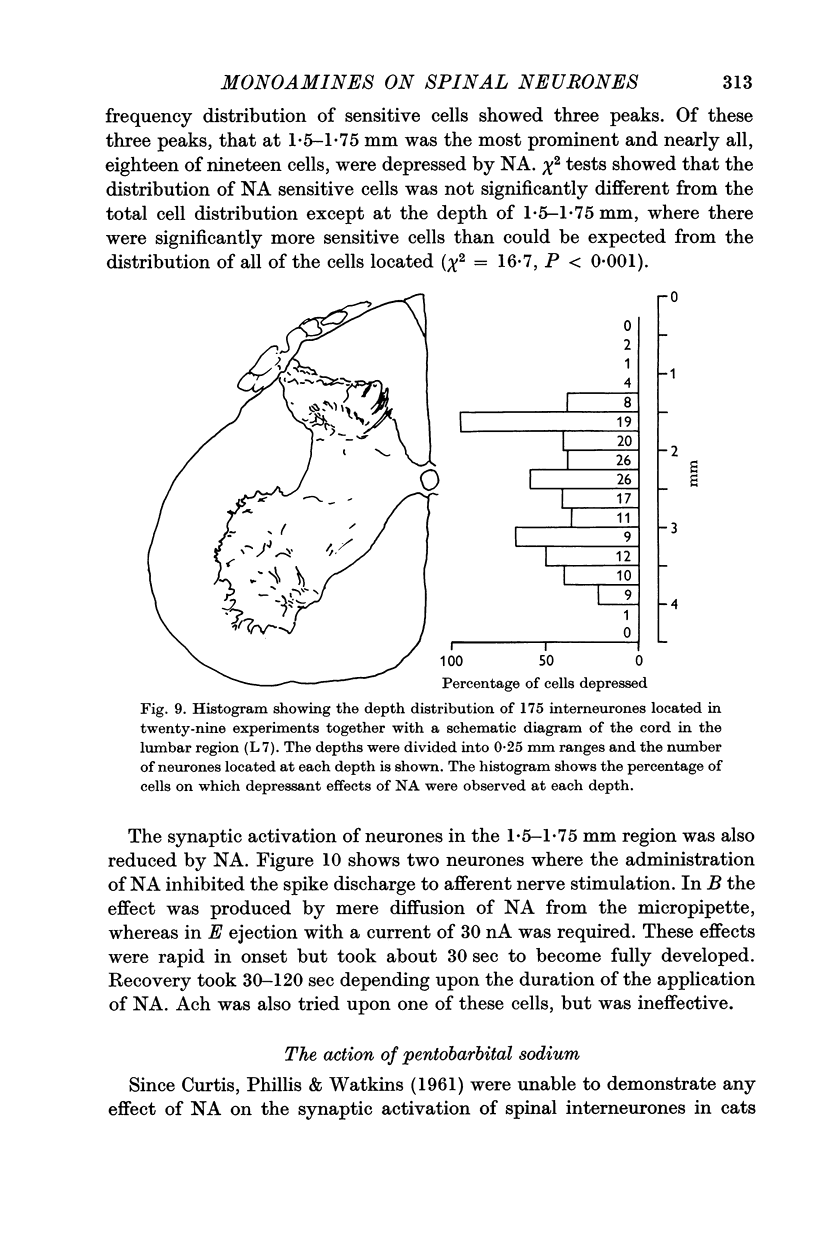
6. There was a correlation between the distribution of NA-sensitive cells and the relative densities of NA-containing terminals in various layers of the grey matter.
7. It was postulated that NA acts as an inhibitory transmitter released from the terminals of descending pathways in the spinal cord. Other possible mechanisms were discussed but lacked experimental support.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., JUKES M. G., LUNDBERG A. SPINAL REFLEXES AND MONOAMINE LIBERATION. Nature. 1964 Jun 20;202:1222–1223. doi: 10.1038/2021222a0. [DOI] [PubMed] [Google Scholar]
- ANDEN N. E., JUKES M. G., LUNDBERG A., VYKLICKY L. A NEW SPINAL FLEXOR REFLEX. Nature. 1964 Jun 27;202:1344–1345. doi: 10.1038/2021344b0. [DOI] [PubMed] [Google Scholar]
- ANDEN N. E., LUNDBERG A., ROSENGREN E., VYKLICKY L. THE EFFECT OF DOPA ON SPINAL REFLEXES FROM THE FRA (FLEXOR REFLEX AFFERENTS). Experientia. 1963 Dec 15;19:654–655. doi: 10.1007/BF02151304. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY P. B., WOLSTENCROFT J. H. ACTIONS OF DRUGS ON SINGLE NEURONES IN THE BRAIN-STEM. Br Med Bull. 1965 Jan;21:15–18. doi: 10.1093/oxfordjournals.bmb.a070349. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., FALCK B., FUXE K., HILLARP N. A. CELLULAR LOCALIZATION OF MONOAMINES IN THE SPINAL CORD. Acta Physiol Scand. 1964 Jan-Feb;60:112–119. doi: 10.1111/j.1748-1716.1964.tb02874.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R. ACTIONS OF DRUGS ON SINGLE NEURONES IN THE SPINAL CORD AND THALAMUS. Br Med Bull. 1965 Jan;21:5–9. doi: 10.1093/oxfordjournals.bmb.a070356. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W. The action of procaine and atropine on spinal neurones. J Physiol. 1960 Aug;153:17–34. doi: 10.1113/jphysiol.1960.sp006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. Cholinergic and non-cholinergic transmission in the mammalian spinal cord. J Physiol. 1961 Sep;158:296–323. doi: 10.1113/jphysiol.1961.sp006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963 Apr;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- MARLEY E. THE ADRENERGIC SYSTEM AND SYMPATHOMIMETIC AMINES. Adv Pharmacol. 1964;3:167–266. doi: 10.1016/s1054-3589(08)61113-8. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]


