Abstract
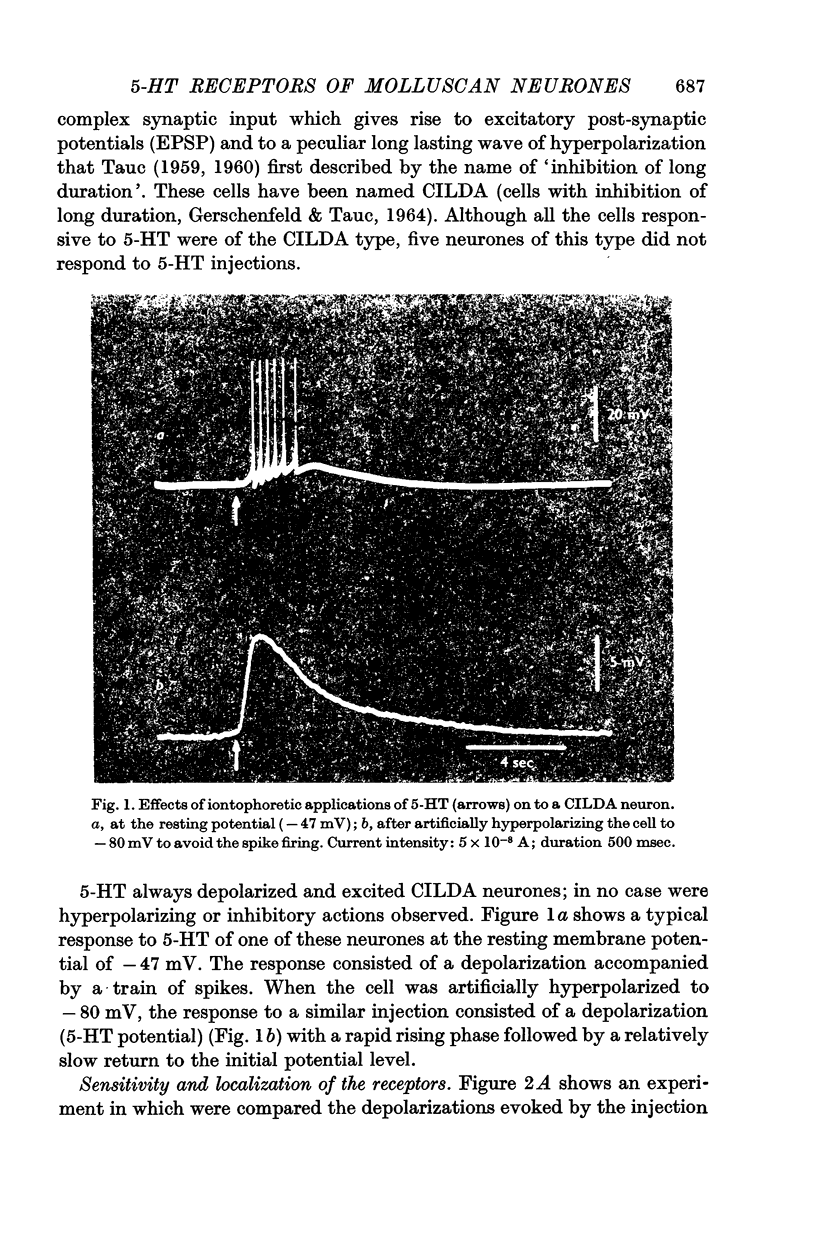
1. 5-Hydroxytryptamine (5-HT) has been iontophoretically applied to the membrane of central neurones of Cryptomphallus aspersa; CILDA neurones (cells with inhibition of long duration) (Gerschenfeld & Tauc, 1964) are the only cells sensitive to 5-HT. The responses to 5-HT is always a depolarization. The CILDA cells studied were also depolarized by ACh.
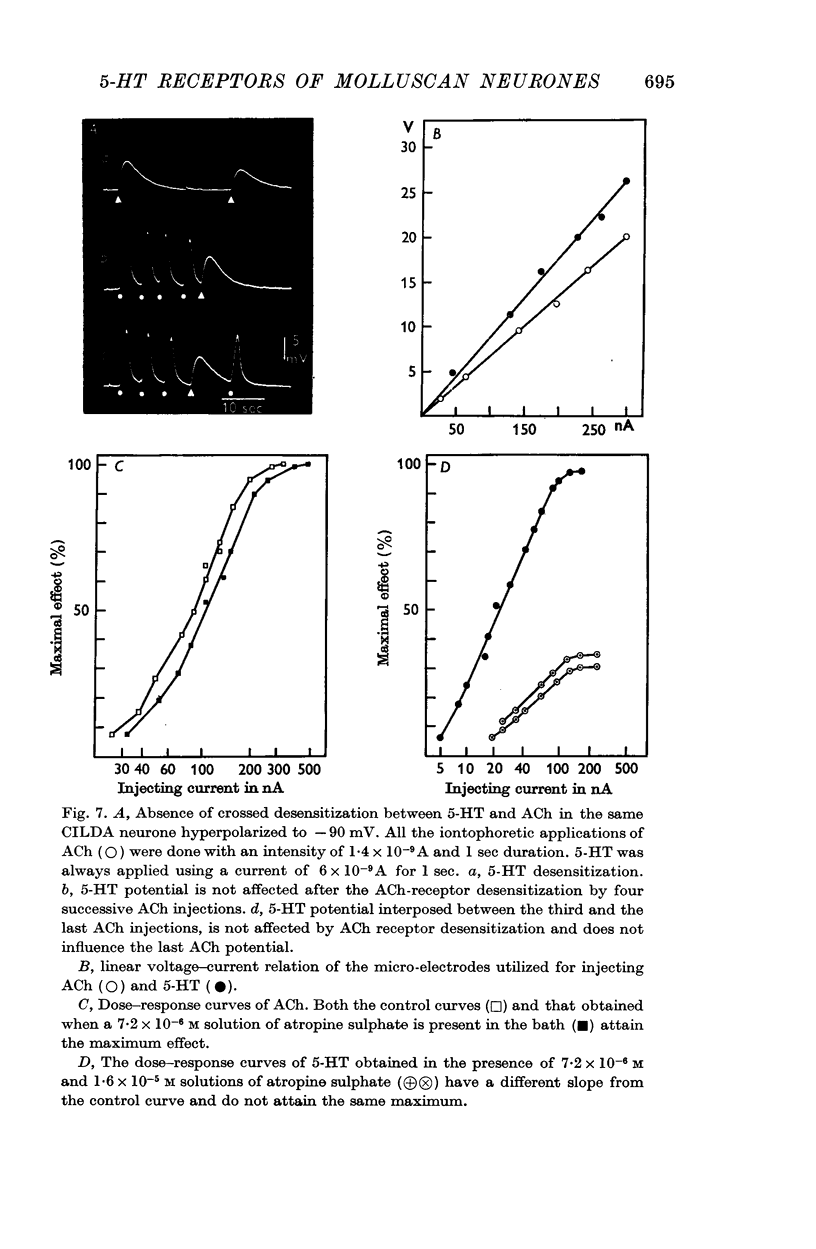
2. From experiments in which pulses of 5-HT and ACh were applied from a double-barrelled micropipette to the CILDA cell soma, it has been calculated that 5-HT and ACh receptors were located at different distances from the injecting micropipette tip. It has also been calculated from the diffusion equation that in the same CILDA cell a 5-HT concentration of 8·2 × 10-9 M and a ACh concentration of 1·3 × 10-8 M caused a similar peak depolarization.
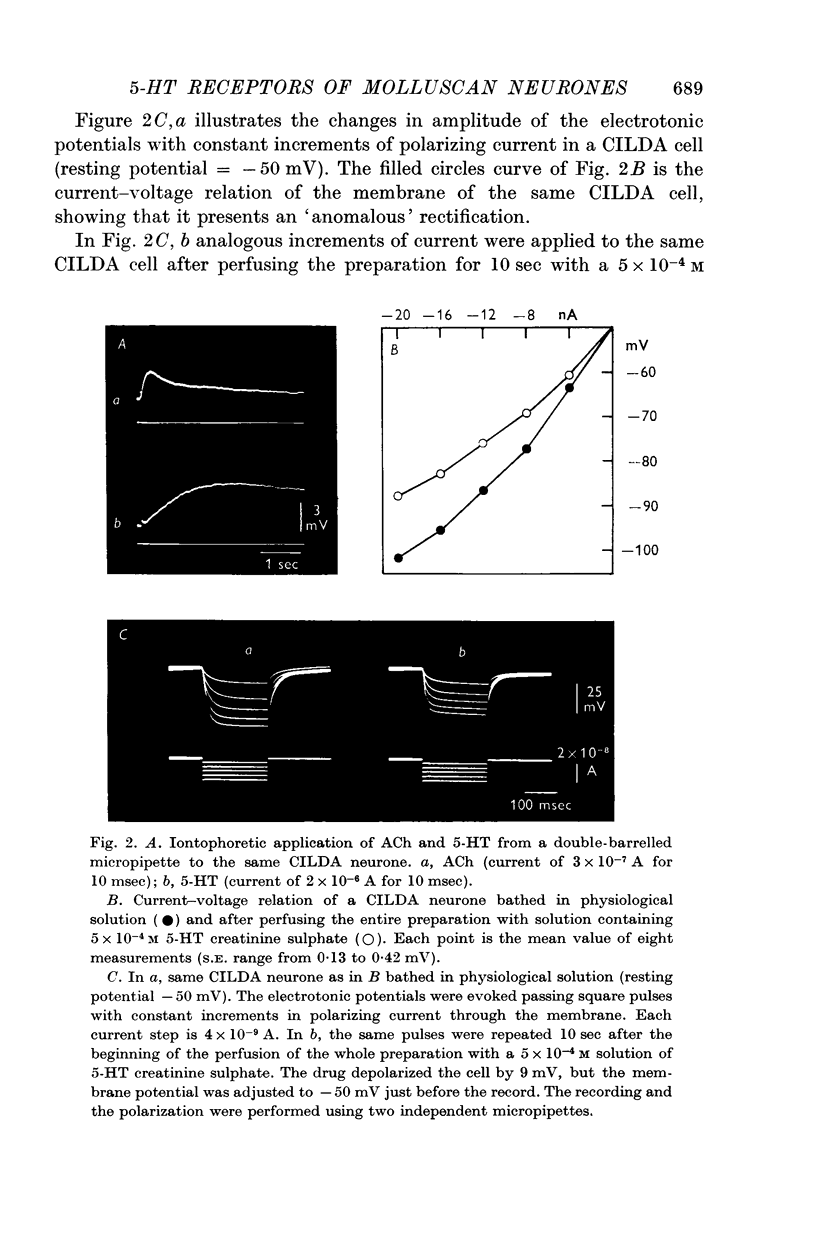
3. CILDA neurones show `anomalous' rectification. 5-HT increases the membrane conductance of CILDA.
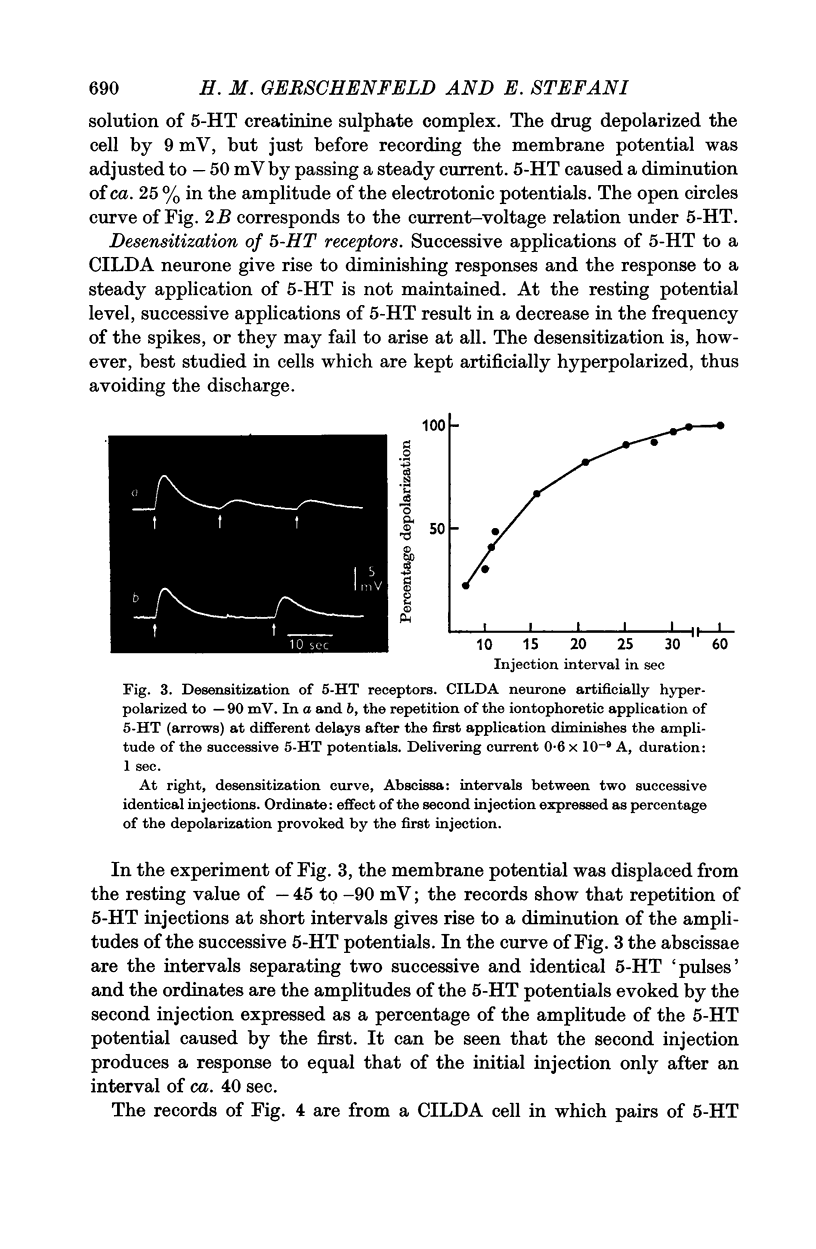
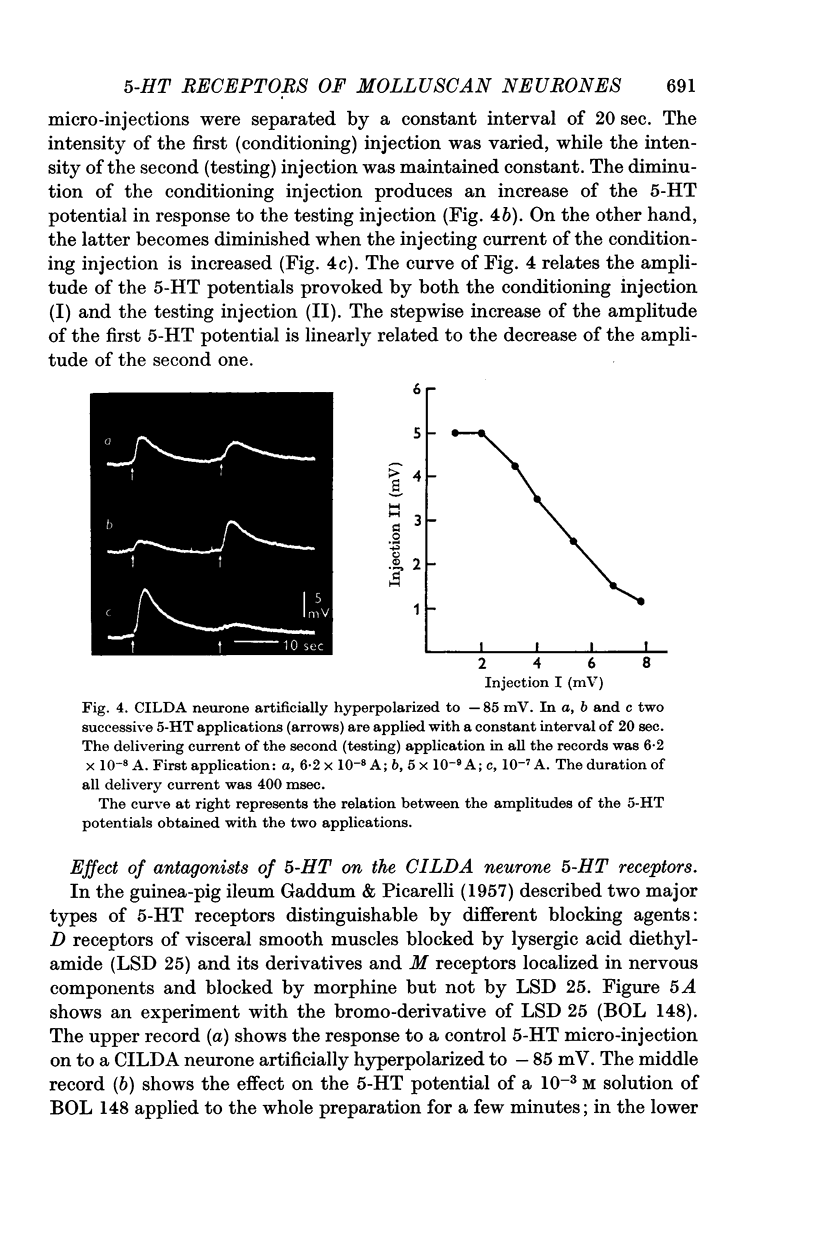
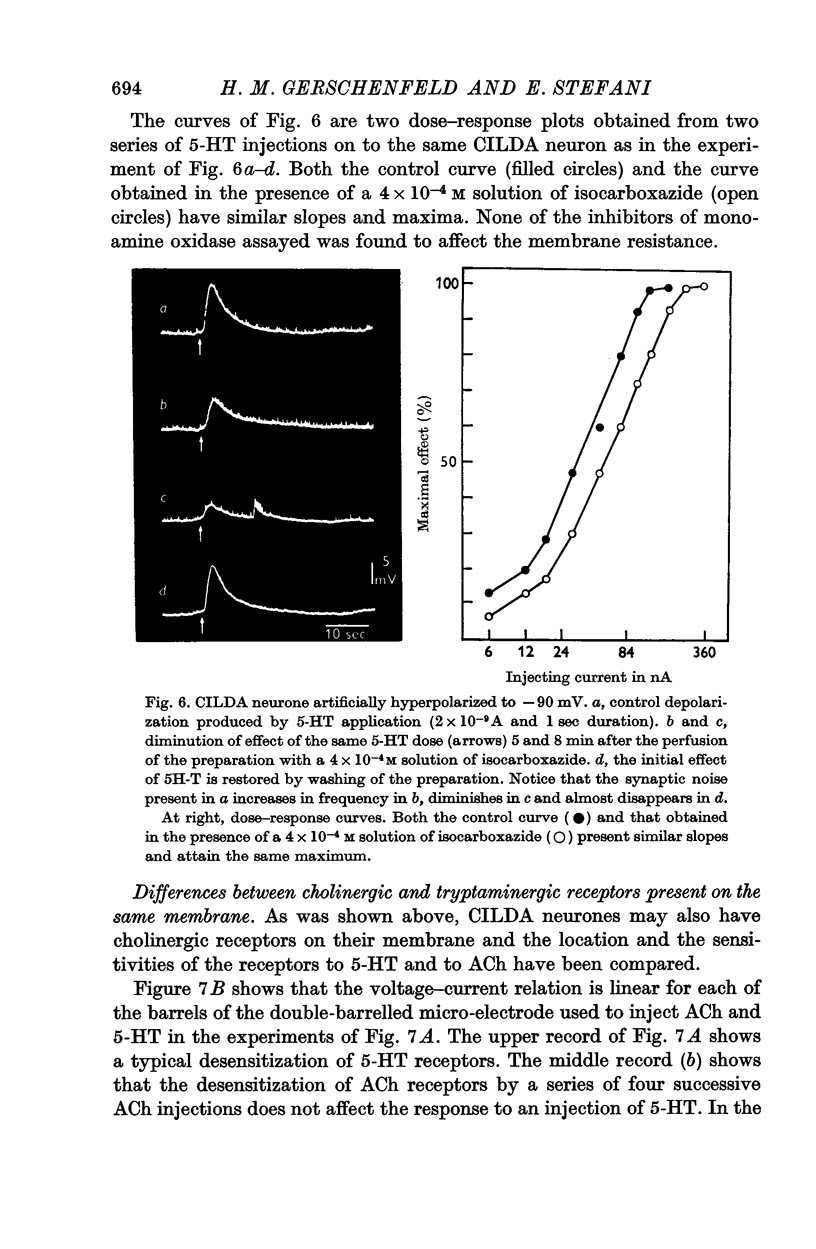
4. 5-HT receptors of CILDA neurone are desensitized by repeated application of 5-HT. The desensitization lasts for ca. 40 sec.
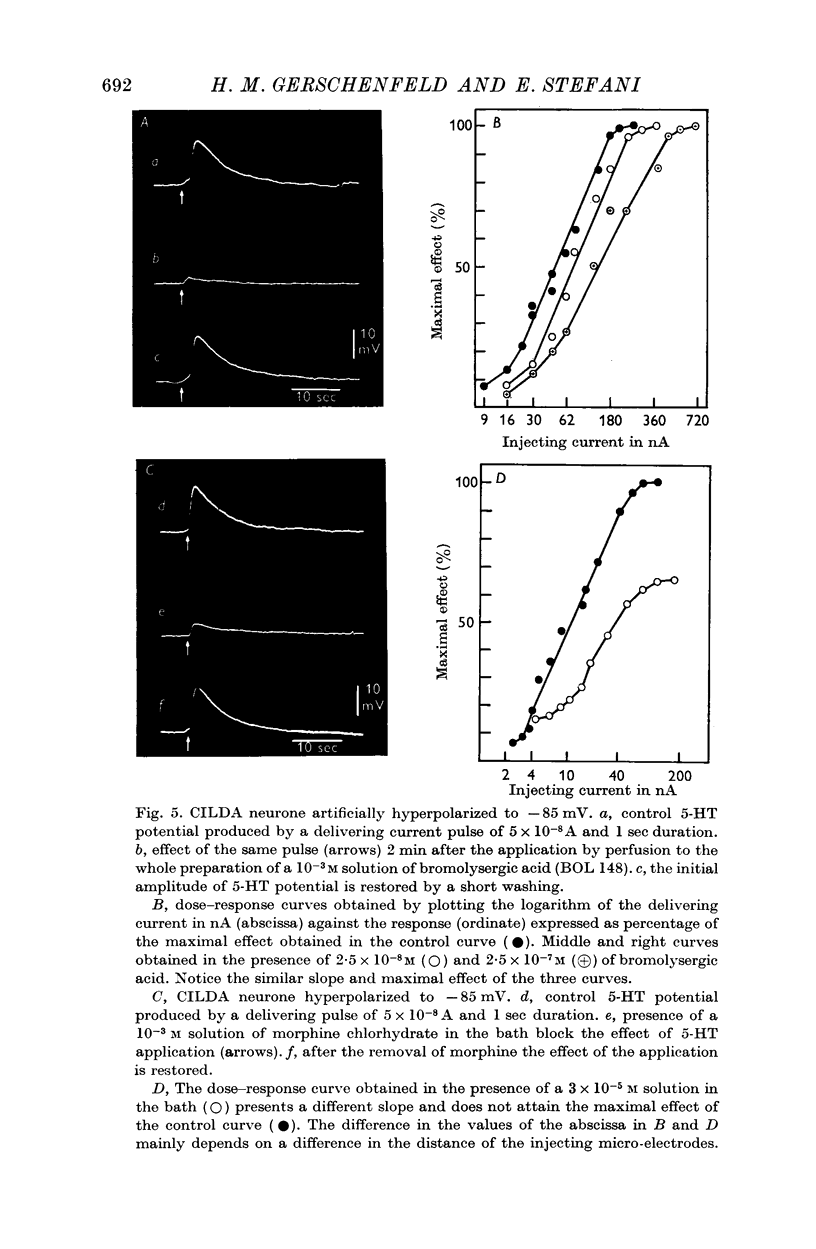
5. 5-HT receptors are blocked by lysergic acid diethylamide and its derivatives. Morphine chlorhydrate blocks them non-competitively.
6. Some inhibitors of monoamine oxidase (trancylpromine, isocarboxazide, iproniazide and nialamide) have been tested. They do not prolong the action of 5-HT, but block the 5-HT receptors.
7. No crossed desensitization between 5-HT and ACh has been observed. Atropine blocks both ACh-receptors and 5-HT receptors, 5-HT receptors appear to be blocked to a greater extent.
8. The data presented support the assumption of a excitatory transmitter role of 5-HT to CILDA neurones, but further evidence is necessary to confirm this hypothesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMIN A. H., CRAWFORD T. B., GADDUM J. H. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol. 1954 Dec 10;126(3):596–618. doi: 10.1113/jphysiol.1954.sp005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGDANSKI D. F., WEISSBACH H., UDENFRIEND S. The distribution of serotonin, 5-hydroxytryptophan decarboxylase, and monoamine oxidase in brain. J Neurochem. 1957;1(3):272–278. doi: 10.1111/j.1471-4159.1957.tb12082.x. [DOI] [PubMed] [Google Scholar]
- BRODIE B. B., SHORE P. A. A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N Y Acad Sci. 1957 Mar 14;66(3):631–642. doi: 10.1111/j.1749-6632.1957.tb40753.x. [DOI] [PubMed] [Google Scholar]
- CARDOT J. CONSID'ERATIONS SUR LE M'ETABOLISME DE LA 5-HYDROXYTRYPTAMINE ET DE LA TRYPTAMINE CHEZ LE MOLLUSQUE HELIX POMATIA. C R Hebd Seances Acad Sci. 1964 Jan 20;258:1103–1105. [PubMed] [Google Scholar]
- CARDOT J., RIPPLINGER J. [Research on the cardio-active indole amines present in nerve tissue of the mollusk Helix pomatia]. J Physiol (Paris) 1963;55:217–218. [PubMed] [Google Scholar]
- CHIARANDINI D. J. A SALINE SOLUTION FOR PULMONATE MOLLUSCS. Life Sci. 1964 Dec;3:1513–1518. doi: 10.1016/0024-3205(64)90098-0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., DAVIS R. Pharmacological studies upon neurones of the lateral geniculate nucleus of the cat. Br J Pharmacol Chemother. 1962 Apr;18:217–246. doi: 10.1111/j.1476-5381.1962.tb01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., KOIZUMI K. Chemical transmitter substances in brain stem of cat. J Neurophysiol. 1961 Jan;24:80–90. doi: 10.1152/jn.1961.24.1.80. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. Cholinergic and non-cholinergic transmission in the mammalian spinal cord. J Physiol. 1961 Sep;158:296–323. doi: 10.1113/jphysiol.1961.sp006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADDUM J. H., PICARELLI Z. P. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957 Sep;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., LASANSKY A. ACTION OF GLUTAMIC ACID AND OTHER NATURALLY OCCURRING AMINO-ACIDS ON SNAIL CENTRAL NEURONS. Int J Neuropharmacol. 1964 Jul;3:301–314. doi: 10.1016/0028-3908(64)90022-x. [DOI] [PubMed] [Google Scholar]
- GERSCHENFELD H. M. Observations on the ultrastructure of synapses in some pulmonate molluscs. Z Zellforsch Mikrosk Anat. 1963;60:258–275. doi: 10.1007/BF00350480. [DOI] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., TAUC L. DIFF'ERENTS ASPECTS DE LA PHARMACOLOGIE DES SYNAPSES DANS LE SYST'EME NERVEUX CENTRAL DES MOLLUSQUES. J Physiol (Paris) 1964 May-Jun;56:360–361. [PubMed] [Google Scholar]
- GERSCHENFELD H., TAUC L. Pharmacological specificities of neurones in an elementary central nervous system. Nature. 1961 Mar 18;189:924–925. doi: 10.1038/189924a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., COTTRELL G. A. ACETYLCHOLINE AND 5-HYDROXYTRYPTAMINE IN THE SNAIL BRAIN. Comp Biochem Physiol. 1963 Jan;8(1):53–63. doi: 10.1016/0010-406x(63)90069-0. [DOI] [PubMed] [Google Scholar]
- MARCHBANKS R. M., ROSENBLATT F., O'BRIEN R. D. SEROTONIN BINDING TO NERVE-ENDING PARTICLES OF THE RAT BRAIN AND ITS INHIBITION BY LYSERGIC ACID DIETHYLAMIDE. Science. 1964 May 29;144(3622):1135–1136. doi: 10.1126/science.144.3622.1135. [DOI] [PubMed] [Google Scholar]
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ DE LORES ARNAIZ G., DE ROBERTIS E. D. Cholinergic and non-cholinergic nerve endings in the rat brain. II. Subcellular localization of monoamine oxidase and succinate dehydrogenase. J Neurochem. 1962 Sep-Oct;9:503–508. doi: 10.1111/j.1471-4159.1962.tb04203.x. [DOI] [PubMed] [Google Scholar]
- SJOERDSMA A., SMITH T. E., STEVENSON T. D., UDENFRIEND S. Metabolism of 5-hydroxytryptamine (serotonin) by monoamine oxidase. Proc Soc Exp Biol Med. 1955 May;89(1):36–38. doi: 10.3181/00379727-89-21707. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., BRUNER J. "Desensitization" of cholinergic receptors by acetylcholine in molluscan central neurones. Nature. 1963 Apr 6;198:33–34. doi: 10.1038/198033a0. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- TAUC L., KANDEL E. R. AN ANOMALOUS FORM OF RECTIFICATION IN A MOLLUSCAN CENTRAL NEURONE. Nature. 1964 Jun 27;202:1339–1341. doi: 10.1038/2021339a0. [DOI] [PubMed] [Google Scholar]
- TAUC L. Sur la nature de l'onde de surpolarisation de longue durée observée parfois après l'excitation synaptique de certaines cellules ganglionnaires de mollusques. C R Hebd Seances Acad Sci. 1959 Jul 15;249(2):318–320. [PubMed] [Google Scholar]
- TWAROG B. M., PAGE I. H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953 Oct;175(1):157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- WELSH J. H., MOORHEAD M. The quantitative distribution of 5-hydroxytryptamine in the invertebrates, especially in their nervous systems. J Neurochem. 1960 Sep;6:146–169. doi: 10.1111/j.1471-4159.1960.tb13460.x. [DOI] [PubMed] [Google Scholar]
- ZIEHER L. M., DE ROBERTIS E. Subcellular localization of 5-hydroxytryptamine in rat brain. Biochem Pharmacol. 1963 Jun;12:596–598. doi: 10.1016/0006-2952(63)90141-2. [DOI] [PubMed] [Google Scholar]


