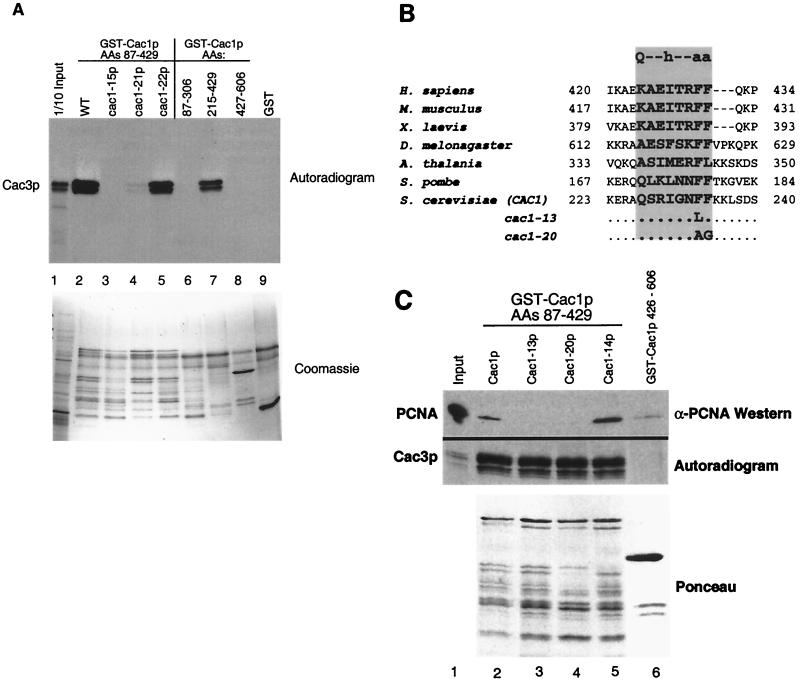
FIG. 2.
Protein binding defects of Cac1p mutants. (A) Cac1-15p does not bind Cac3p in vitro. GST fusion proteins (amino acids 87 to 429 of Cac1p, Cac1-15p, Cac1-21p, or Cac1-22p, amino acids 87 to 306, 215 to 429, or 427 to 606 of Cac1p, or an unfused GST control) were incubated with in vitro-translated Cac3p and washed extensively in the presence of 500 mM NaCl. Bound proteins were eluted with SDS sample buffer, separated by SDS-12.5% PAGE, and visualized by autoradiography. The input (lane 1) contains 1/10 of the amount of translation product input into the binding assays. The upper panel labeled autoradiogram shows in vitro-translated Cac3p, and the lower panel labeled Coomassie shows the input GST fusion proteins for the same experiment. The GST-Cac1p proteins were susceptible to proteolysis, resulting in the pattern of bands shown in the lower panel. (B) Evolutionary conservation of the consensus PCNA-binding motif in CAC1 homologues from human (GenBank accession number XM009408), mouse (NM013733), Xenopus laevis (AF222339), Drosophila melanogaster (AF367177), Arabidopsis thalania (AB027229), Schizosaccharomyces pombe (AL034463), and S. cerevisiae (NP015343). The consensus PCNA-binding motif is shown at the top of the shaded rectangle. Mutations in the cac-13 and cac1-20 alleles are indicated. Residues comprising the PCNA-binding motif are in bold. (C) Cac1-13p and Cac1-20p are defective in PCNA binding. GST fusion proteins (amino acids 87 to 429 of Cac1p, Cac1-13p, Cac1-14p, or Cac1-20p [lanes 2 to 5] or amino acids 427 to 606 of Cac1p [lane 6]) were incubated with purified recombinant yeast PCNA or in vitro-translated Cac3p. Samples were washed extensively in the presence of 500 mM NaCl, and bound proteins were eluted with SDS sample buffer and separated by SDS-15% PAGE. Cac3p was visualized by autoradiography, and PCNA was visualized by immunoblotting. The lower panel labeled Ponceau shows the input GST fusion protein for the PCNA coprecipitation experiment. For Cac3p, the input lane contains 1/30 of the amount of translation product input into the binding assays; for PCNA, the input lane contains 1/10 of the amount input (lane 1).