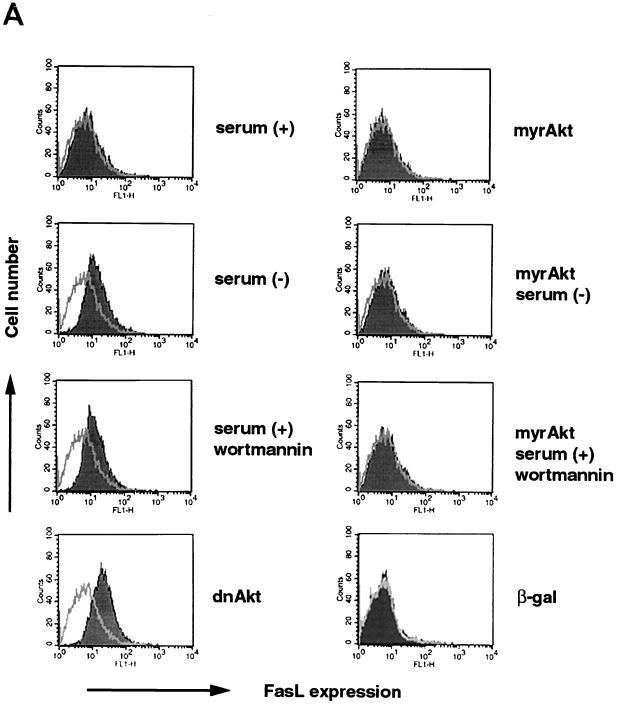
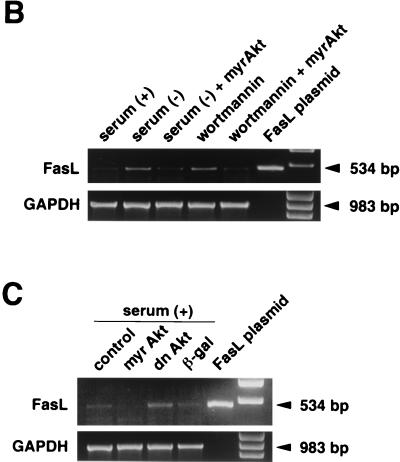
FIG. 1.
FasL expression is induced by serum deprivation or suppression of PI 3-kinase/Akt signaling. (A) Cell surface expression of FasL on human VSMC was determined by flow cytometry. VSMC were cultured with or without 10% serum, in the presence or absence of wortmannin (200 nM) for 48 h. Alternatively, cells were incubated with an adenoviral construct expressing dominant-negative Akt (dnAkt) (MOI = 100) for 48 h prior to analysis. Other cultures were preinfected with an adenoviral construct expressing constitutively active Akt (myrAkt) (MOI = 100) for 24 h prior to exposure to wortmannin or serum deprivation medium for 48 h. After treatments, cells were detached from the culture plate with 0.5% EDTA, incubated with anti-human FasL antibody (hamster IgG; 4H9), and stained with FITC-conjugated anti-hamster antibody (shaded curve). Hamster IgG was used as a negative control (open curve). Representative data from three replicate experiments are shown. PI 3-kinase (B) and Akt (C) signaling regulates FasL mRNA expression in human VSMC. Semiquantitative RT-PCR analysis was performed on FasL and GAPDH transcripts. RNA was isolated by a guanidine isothiocyanate-acid phenol method and reverse transcribed. A plasmid encoding human FasL cDNA served as a positive control for the FasL RT-PCR signal. The GAPDH signal shows uniformity of the RT-PCR analysis. Marker bands appear in the lanes on the far right. Representative data from two replicate experiments are shown in each panel.