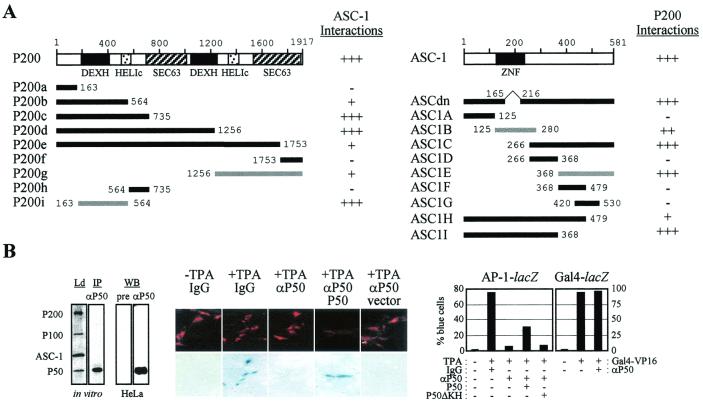
FIG. 7.
P200-ASC-1 interaction interfaces and essentiality of P50 in AP-1 transactivation. (A) A combination of fusions of B42-ASC-1 and LexA to wild-type P200 or P200 deletion mutants (left panel) and fusions of B42 to the wild-type ASC-1 or ASC-1 deletion mutants and LexA-P200 (right panel) were transformed into a yeast strain containing an appropriate lacZ reporter gene, as described previously (2). None of the LexA-P200 constructs exhibited autonomous transactivation function (data not shown). +++, strongly blue colonies after 2 days of incubation; ++, light blue colonies after 2 days of incubation; +, light blue colonies after more than 2 days of incubation; −, white colonies. Gray bars, deduced interaction domains. Two copies of DExH-type helicase domains, the helicase superfamily C-terminal domains (HELIc), and SEC63 domains of unknown function within P200 as well as the zinc finger (ZNF) domain of ASC-1 are indicated. (B) (Left) Specificity of the P50 antibody was demonstrated by specific immunoprecipitation (IP) of P50 from a mixture of in vitro-translated and radiolabeled constituents of the ASC-1 complex as well as specific detection of P50 from HeLa nuclear extract in Western analysis (WB). Ld and pre, 20% of the reaction mixture and preserum, respectively. (Middle) Photographs of FITC-stained injected cells (top) and the corresponding pattern of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-thiogalactopyranoside) staining (bottom) with microinjection of either control IgG or anti-P50 IgG. The presence or absence of 0.1 μM TPA is indicated. (Right) The number of cells that express lacZ relative to the total number of FITC-positive cells microinjected with either control IgG or anti-P50 IgG. The presence or absence of TPA (0.1 μM) and a P50, P50ΔKH, or Gal4-VP16 expression vector is indicated. The reporter constructs were AP-1-lacZ and Gal4-lacZ. Experiments were repeated twice with similar results (error range, 5 to 10%), with >200 cells injected.