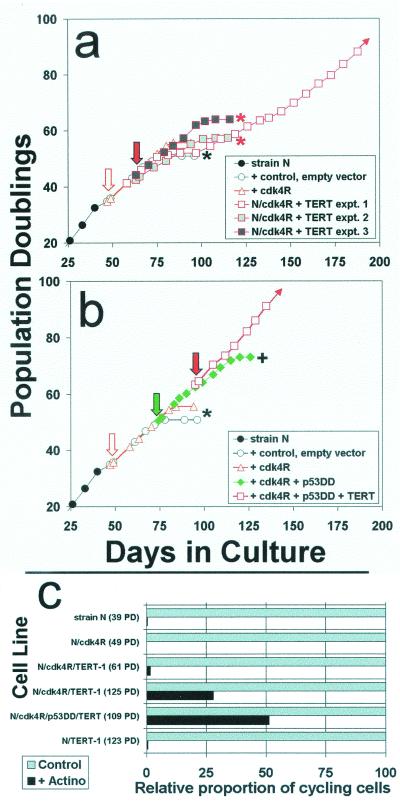
FIG. 6.
Engineering keratinocytes to resist p16INK4A inhibition and to express hTERT is not sufficient to immortalize them. (a) Strain N cells were first transduced to express cdk4R. Then, in three separate experiments, aliquots of the N/cdk4R population were transduced to express hTERT and serially passaged. Downward arrows indicate timing of the respective transductions. Asterisks indicate complete senescence of the cell population. + indicates evasion of senescence and growth for a substantially extended life span. Note that in all three experiments, the cdk4R/TERT double transductants senesced at the same time or only slightly later than strains N and N/cdk4R. Only experiment 1 yielded a rare immortalized variant. (b) In another experiment, the same N/cdk4R line shown in panel a was first transduced to express p53DD and then to express hTERT. Note that the N/cdk4R/p53DD/TERT cells immediately exhibited an immortalized phenotype, without any inflection in the life span plot that would have suggested outgrowth of a rare variant. (c) Loss of the DNA damage response by rare variants of N/cdk4R/TERT-1 cells that evaded senescence and became immortalized. Cells growing in K-sfm medium were untreated or pretreated with low concentrations of actinomycin D tointroduce DNA strand breaks, received BUdR for the final 20 h before fixation, and were immunostained for BUdR as described in the text. From 150 to 500 cells in at least four randomly selected fields were scored for labeled nuclei. The labeling index (percent cycling cells) of control cultures was 70 to 80% for all lines shown. These control values were normalized to 100% (gray bars), and the relative numbers of labeled nuclei in the actinomycin-treated culture of each line (black bars) are shown in the graph. Note that the parent primary line strain N cells, presenescent N/cdk4R cells, and early N/cdk4R/TERT-1 cells exhibited the normal DNA damage response of cell cycle arrest. In contrast, the later-passage, rapidly dividing, immortalized N/cdk4/TERT-1 cultures and N/cdk4R/p53DD/TERT cultures contained a substantial frequency of cells that were not arrested and continued to cycle following DNA damage. Note also that N/TERT-1, a line previously shown to be deficient in p16 expression but having normal p53 function (17), displayed a normal DNA damage response, demonstrating that p16 deficiency and telomere stabilization alone do not impair this response.