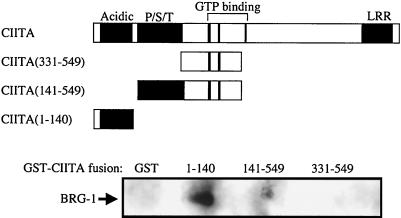
FIG. 6.
Affinity isolation of BRG-1 by the N-terminal portion of CIITA. GST, or GST-CIITA fusion proteins were expressed in E. coli and purified with glutathione-Sepharose. The recombinant proteins were combined with nuclear extract from RJ2.2.5 cells and allowed to interact overnight. Following extensive washing, the bound proteins were analyzed by Western blot analysis with anti-BRG-1 antiserum. The top of the figure is a diagram of the regions of CIITA used in the GST fusion proteins. Acidic, region rich in aspartic and glutamic acid residues (amino acids 26 to 140); P/S/T, region rich in proline, serine, and threonine residues (amino acids160 to 319); LRR, leucine-rich region (amino acids 985 to 1086).