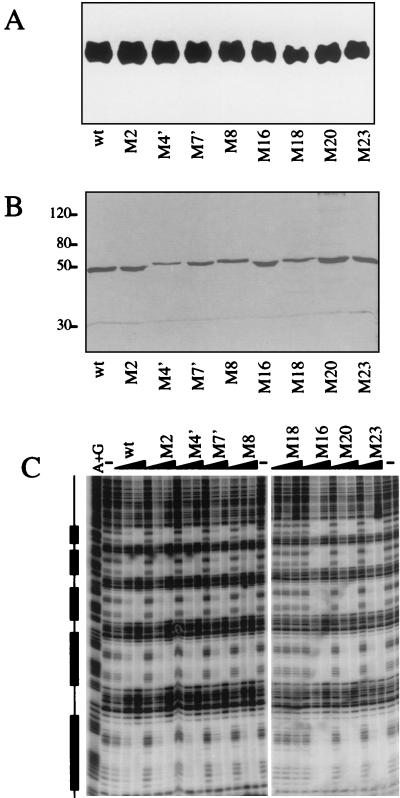
FIG. 3.
Effects of the mutations on protein stability and DNA-binding activity. (A) Mutations do not affect levels of nuclear Dorsal protein. S2 cells (50 ml) were transiently transfected with 2 μg of wild-type (wt) Dorsal380 or the indicated mutant forms of Dorsal380. Cells were harvested 2 days posttransfection, and nuclear extracts were prepared. The proteins were resolved by SDS-10% PAGE, transferred to a polyvinylidene difluoride membrane, and probed with anti-Dorsal antibody. (B) Purification of recombinant Dorsal mutants. Flag-tagged Dorsal380 or the indicated Dorsal380 mutants were immunopurified to homogeneity from Sf9 cells infected with recombinant baculoviruses. Proteins were resolved by SDS-10% PAGE and silver stained. (C) With one exception, the mutations do not alter DNA-binding activity. The purified proteins shown in panel B were assayed for binding to the DE5 enhancer by a DNase I footprinting assay. The boxes to the left indicate the Dorsal binding sites. Each protein was assayed at 2, 10, and 50 ng. Lane A+G, A+G chemical sequencing ladder; lane −, no-protein control.