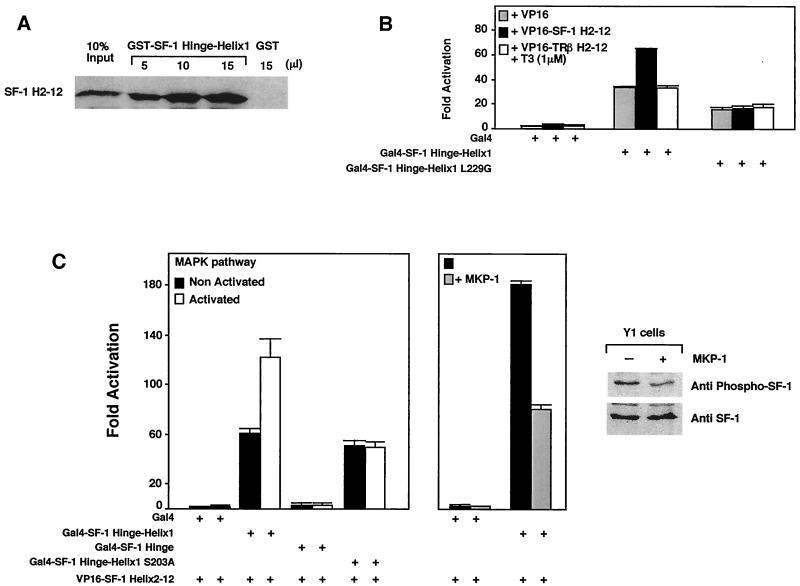
FIG. 3.
Phosphorylation of SF-1 enhances recruitment of the LBD. (A) In vitro assembly of increasing amounts of purified recombinant GST-SF-1 hinge-helix 1 protein with the in vitro-transcribed and translated [35S]methionine-labeled remainder of the LBD protein (SF-1 H2-12) were assessed by a standard GST pulldown assay. Ten percent of the labeled input is shown as indicated (10% input), along with a single pulldown with control GST protein (GST). (B) Association of SF-1 hinge-helix 1 with the remainder of the LBD was tested in a mammalian two-hybrid experiment using NIH 3T3 δRaf-1:ER cells. The activation domain VP16 fused to SF-1 helices 2 to 12 (VP16-SF-1 H2-12) and the SF-1 hinge-helix 1 fused to the Gal4 binding domain were cotransfected with the Gal4-responsive luciferase reporter pGAL-RE-TK in δRaf-1:ER cells for 24 h. VP-16 fused to helices 2 to 12 of TRβ was also used in these experiments in the presence of T3 (1 μM). Similar results were obtained with the mouse Y1 adrenocortical cell line, and assembly was shown to be dependent on VP16 (data not shown). (C) MAPK pathway activation increases helix assembly of SF-1 in vivo. The luciferase activity of the pGAL-RE-TK (Gal4 reporter) is shown for a mammalian two-hybrid expression system containing VP16-SF-1 H2-12 with different deletions, and mutants of SF-1 hinge-helix 1 fused to Gal4 DBD were cotransfected in the δRaf-1:ER or in Y1 cells for 24 h. Activity was also measured in an S203A mutant harboring a nonphosphorylatable alanine residue. To activate the MAPK pathway, δRaf-1:ER cells were treated with 10−5 M β-estradiol for 5 h. To inhibit the MAPK pathway, the MAPK inhibitor MAPK phosphatase (MPK-1) expression vector was cotransfected in Y1 cells grown in standard growth media. The degree of SF-1 phosphorylation was evaluated by using an S203-phosphospecific SF-1 antibody compared with signals obtained with a SF-1 antibody, with (+) our without (−) transfection of the MPK-1. A representative Western blot is shown in the far-right panel.