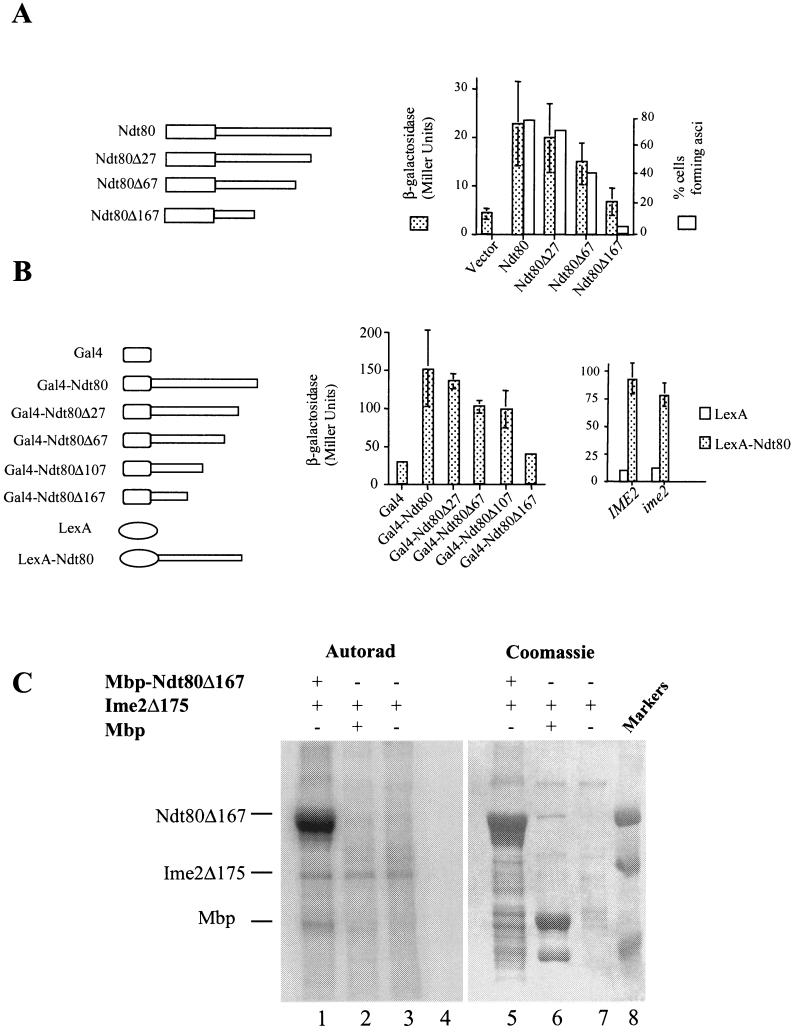
FIG.6.
The C terminus of Ndt80 encodes a transactivation domain. (A) The endogenous NDT80 in a diploid yeast strain was replaced by a version of NDT80 encoding either full-length Ndt80 or the indicated C-terminal deletion (Δ27, Δ67, or Δ167). These strains also harbored an SPS4-LacZ reporter gene. The ability of these mutant versions of NDT80 to promote middle sporulation gene expression was assayed by the β-galactosidase activity induced after 6 h in SPM and is expressed in Miller units. The ability of the mutant versions of NDT80 to promote and scored for the formation of asci. (B) IME2 is not required for the function of the NDT80 trans activation domain. The C terminus of NDT80 or the indicated C terminally deleted versions of NDT80 were fused to the Gal4 DNA binding domain and expressed in vegetatively growing cells under the regulation of a CUP1 promoter. β-Galactosidase activity expressed from an integrated GAL1-LacZ reporter gene was measured and is displayed in Miller units. Similarly, the entire Ndt80 C terminus (amino acids 426 and 627) was fused to LexA and expressed under the regulation of a CUP1 promoter in IME2/IME2 or ime2/ime2 diploid cells. Both strains harbor an integrated 8×Lexo-LacZ reporter gene. β-Galactosidase activity induced by the LexA fusions was assayed after the cells had been in SPM for 5 h. (C) Ime2 phosphorylates Ndt80 lacking its transactivation domain. An Mbp-Ndt80 fusion lacking 167 amino acids from its C terminus was produced in E. coli and used as a substrate for immunoprecipitated Ime2Δ175. Lanes 1 to 4 show an autoradiogram (Autorad) of the kinase assay, while lanes 5 to 8 show Coomassie staining of the same gel. Ime2Δ175 kinase activity was assayed against Mbp-Ndt80Δ167 (lanes 1 and 5), Mbp1 (lanes 2 and 6), or no added substrate (lanes 3 and 7). Molecular weight markers are included in lane 8.