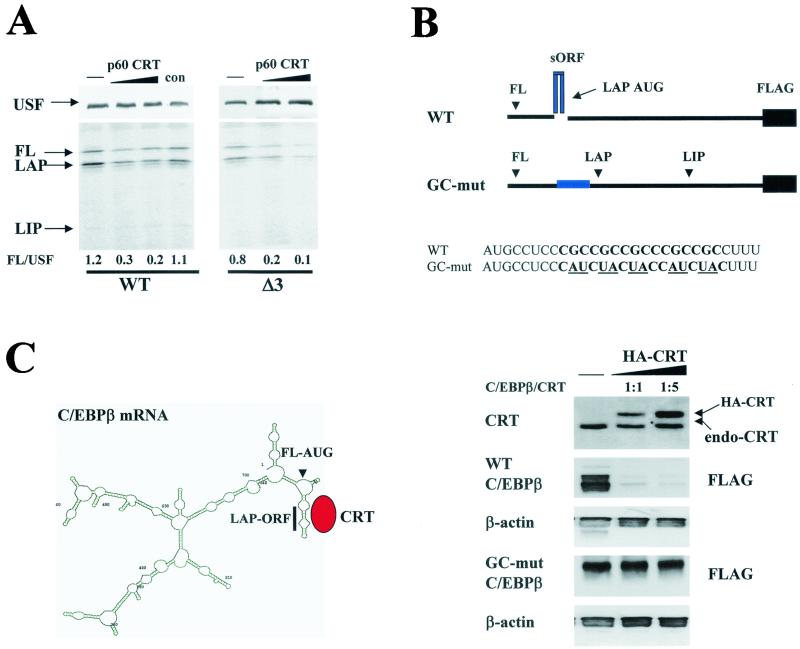
FIG. 6.
CRT inhibits translation of C/EBPβ proteins. (A) CRT inhibits translation of C/EBPβ mRNA in a cell-free translation system. Purified p60/CRT (Fig. 4) was added to rabbit reticulocyte lysate programmed with USF (control) mRNA and with wild-type (WT) C/EBPβ or Δ3 (mutation of the third AUG codon) mutant C/EBPβ mRNA. The translation mixtures were divided, and USF and C/EBPβ were precipitated with specific antibodies from the same reactions. The levels of C/EBPβ isoforms were calculated as ratios to that of USF. The positions of full-length (FL), LAP, and LIP isoforms are shown. con, an UnoQ fraction which does not contain CRT. (B) CRT inhibits translation of C/EBPβ in cultured cells. (Top) Structure of the 5′ regions of wild-type and GC-mut C/EBPβ mRNAs and mutations incorporated in the 5′region. Replacements of GC islands (shown in bold) with AU or UA (underlined) abolish the formation of an SL structure within GC-mut C/EBPβ mRNA. Both wild-type and mutant C/EBPβ mRNAs were fused to FLAG to distinguish expression of endogenous and exogenous C/EBPβ proteins. The positions of the AUG codons for isoforms of C/EBPβ are shown. The sequences of wild-type and mutant 5′ regions of C/EBPβ mRNA are shown below. (Bottom) Wild-type C/EBPβ-FLAG and GC-mut-C/EBPβ-FLAG were cotransfected with increasing amounts of plasmid expressing HA-CRT into HT1080 cells; 18 h after transfection, total proteins were isolated and examined by Western blotting with antibodies to CRT or to FLAG. The positions of HA-CRT and an endogenous CRT (endo-CRT) are shown. The membranes were reprobed with antibodies to β-actin. (C) Hypothetical model for CRT-dependent inhibition of C/EBPβ translation. The predicted secondary structure of full-length C/EBPβ mRNA revealed a stable SL organization of the CRT binding site (sORF). We suggest that CRT binds to the GC-rich stem of the 5′ region of C/EBPβ mRNA and blocks initiation from the first and second AUG codons.