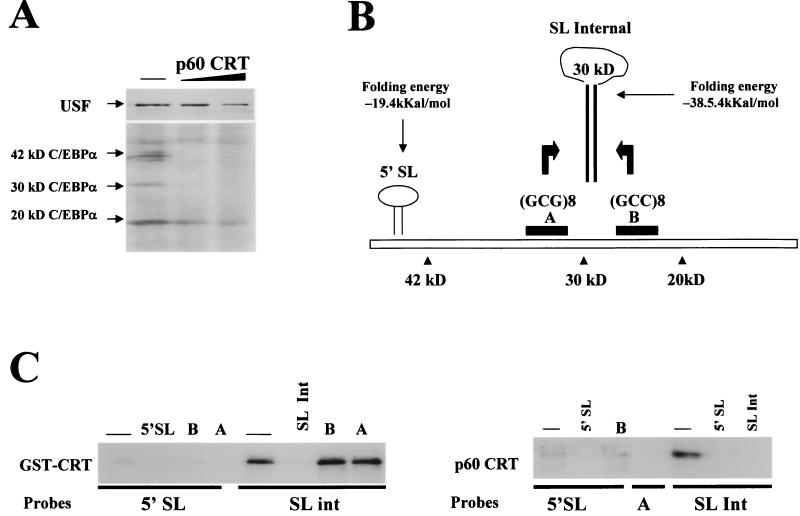
FIG. 7.
CRT binds to C/EBPα mRNA and represses translation of the 42- and 30-kDa isoforms of C/EBPα. (A) Purified p60/CRT represses translation of C/EBPα in a cell-free translation system. Experiments were performed as described in the legend to Fig. 6A. C/EBPα and USF were translated simultaneously in the presence of increasing amounts of CRT and then immunoprecipitated with specific antibodies. The positions of the 42-, 30-, and 20-kDa isoforms are shown. (B) Putative SL structures within C/EBPα mRNA. RNA secondary-structure predictions revealed two putative SL structures with GC-rich stems. The predicted SL structure in the 5′ region is located upstream of the first AUG codon. Within the coding region, the GCG repeat (region A) and the GCC repeat (region B) can form a stable secondary structure. (C) CRT binds preferentially to the stem structure within the internal SL of C/EBPα mRNA. (Left) UV cross-link reaction using activated GST-CRT (see Materials and Methods) and the 5′ SL or with SL int probes. Cold competitors (shown at the top) were added to the reaction mixture prior to the addition of probe. A and B are single-stranded RNA oligomers covering regions A (GCG repeat) and B (GCC repeat), respectively. CRT does not interact with the single-stranded A and B RNA oligomers but binds strongly to a double-stranded structure formed by annealing these probes (SL int). (Right) p60/CRT was incubated with the 5′ SL or SL int probe in the absence or the presence of RNA competitors (shown at the top) and analyzed by UV cross-link assay.