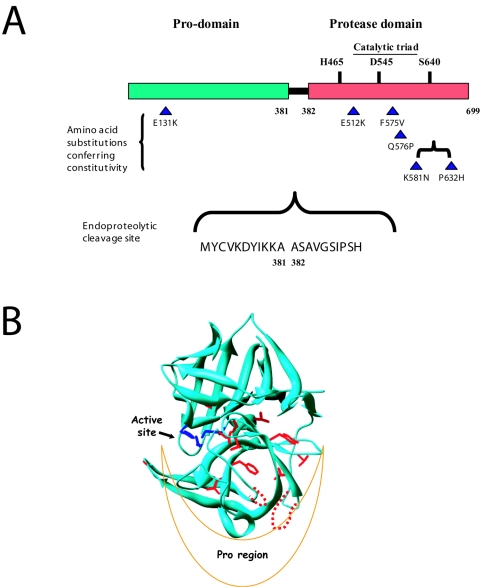
FIG. 4.
Structural features of the Ssy5 protease. (A) The positions of amino acid substitutions in the constitutive SSY5 mutants constructed in the present work are indicated by triangles together with the position of the E512K substitution in SSY5-6 reported previously (28). The proposed (1, 2) catalytic triad, as conserved in chymotrypsin-like serine proteases, is also indicated. The site of endoproteolytic cleavage, between amino acid residue positions 381 and 382, is indicated with flanking residues. (B) The structure of α-lytic protease from Lysobacter enzymogenes (PDB code 1SSX) is shown in ribbon representation, with the catalytic triad (in blue) and identical residues aligned by PHYRE (in red) shown as sticks. The loops predicted to be longer in Ssy5p and containing four of the substitutions encoded by the constitutive mutations are shown as dashed lines in red.