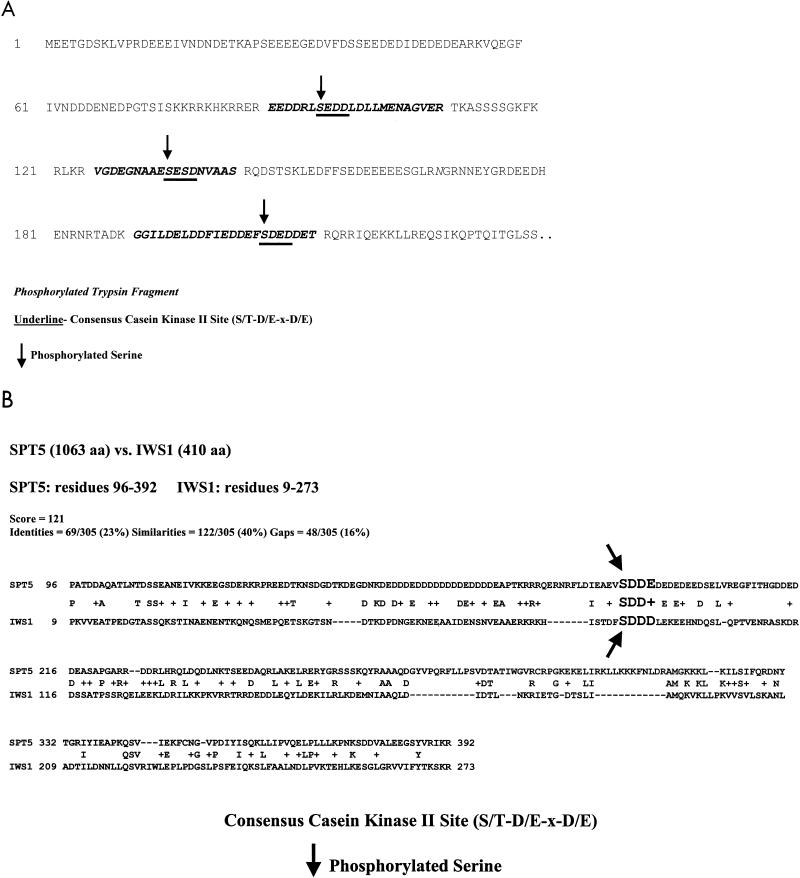
FIG. 2.
Detection of phosphorylation on putative CKII sites in Spt5, Spt6, and Iws1. (A) Identification of phosphorylation sites in Spt6. Tandem mass spectrometry was used to identify three tryptic fragments in the N-terminal region of Spt6 that were phosphorylated on serine residues (serines 95 [SEDD], 135 [SESD], and 207 [SDED]). Each phosphorylated serine residue in Spt6 was at the beginning of a consensus CKII site. (B) Phosphorylation of a conserved serine present in both Spt5 and Iws1. Mass spectrometry successfully detected a phosphorylation site on serine 89 (SDDD) of Iws1 as well as on serine 188 (SDDE) of Spt5; each of these occurs at the start of a putative CKII site. The acidic N-terminal regions of Spt5 and Iws1 have sequence similarity, and their phosphorylated serine residues align perfectly. The regions compared have 23% identity and 40% similarity.