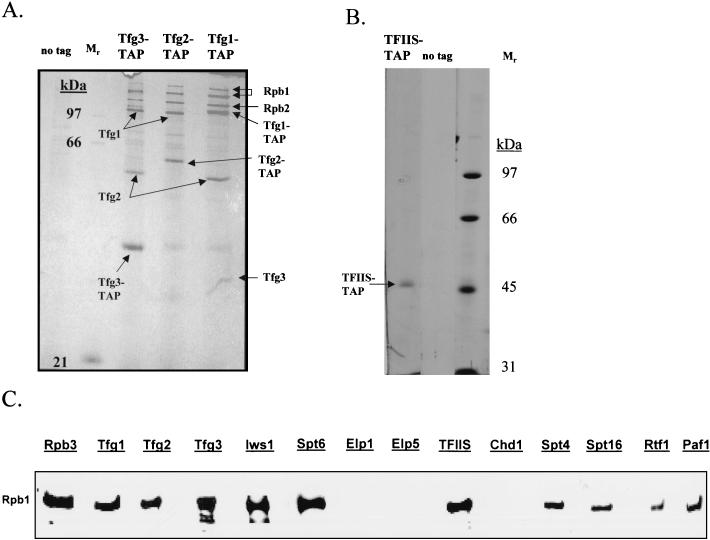
FIG. 4.
TAPs of TFIIF and TFIIS and detection of RNAPII interactions by Western blot analysis. (A) Purification of TFIIF by using TAP-tagged versions of Tfg1, Tfg2, and Tfg3. Purified proteins were analyzed by SDS-PAGE, silver staining, and mass spectrometry. Purification of any of the three subunits of TFIIF in buffers containing 150 mM NaCl results in isolation of all three components of TFIIF as well as a substoichiometric amount of RNAPII. (B) Tagging and purification of TFIIS. Purification of TFIIS (encoded by the DST1 gene) in buffers containing 100 mM NaCl resulted in the isolation of predominantly TFIIS. Further development of the silver stain revealed only background proteins and none that had copurified with TFIIS. (C) Western blot analysis of transcription elongation factors purified in the presence of 100 mM NaCl by using monoclonal antibody 8WG16, which binds to the CTD of the largest subunit of RNAPII, Rpb1. As a positive control, RNAPII was purified from a strain with a TAP tag on its third-largest subunit, Rpb3. Equal volumes of the column eluates from the various purifications were loaded onto the gel, except that approximately 10 times less of the Rpb3 purification was loaded. RNAPII, in the presence of 100 mM NaCl, copurified with TFIIF (Tfg1, -2, and -3), Spt4/Spt5, Spt6/Iws1, TFIIS, Spt16, and Paf1/Rtf1, but not with Elongator (Elp1 and Elp5) or Chd1.