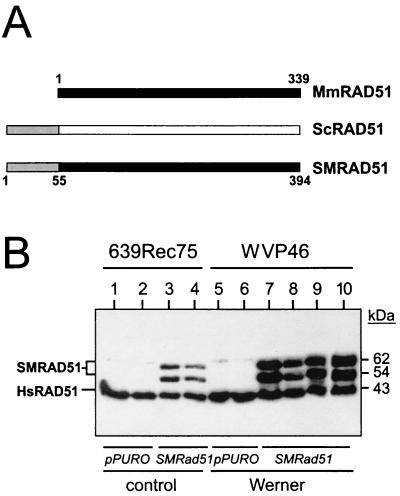
FIG. 3.
Dominant-negative mammalian RAD51 protein and expression in stable cell lines. (A) Mus musculus (Mm), S. cerevisiae (Sc), and chimeric Mm-Sc (SM) RAD51 proteins (the numbers indicate amino acid residues) (22). Filled and open boxes represent the M. musculus and S. cerevisiae RAD51 open reading frames, respectively. Shaded segments represent the 55 N-terminal amino acid residues of yeast RAD51 that have been fused in frame to mouse RAD51 to generate the chimeric SMRAD51 protein. (B) Western blot analysis of expression of endogenous (HsRAD51) or stably expressed SMRAD51 proteins in representative control (639Rec75) or WRN (WVP46) SV40-transformed fibroblast-derived sublines (35). Lanes 1, 2, 5, and 6 were clonally derived from control plasmid (pPURO) transfections. The two SMRAD51 protein bands arise from alternative initiation or stable degradation (22). Multiple independent sublines were generated for use in subsequent experiments.