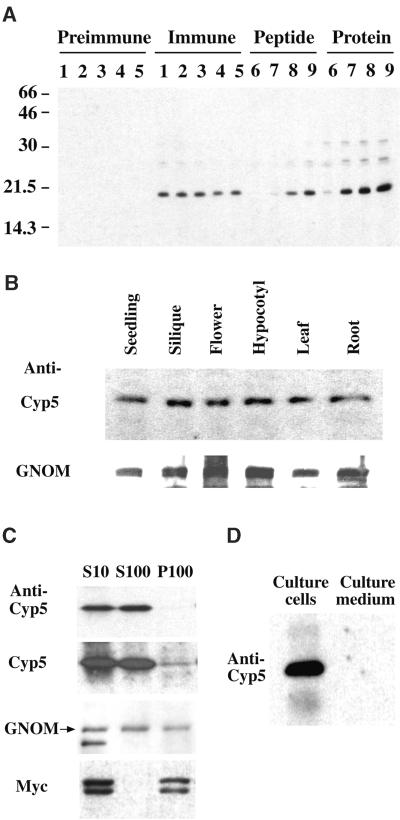
Figure 8.
Expression and Subcellular Localization of Cyp5 Protein.
(A) Specificity of polyclonal anti-Cyp5 peptide antiserum. Shown is an immunoblot of total silique protein. Preimmune and immune serum dilutions are as follows: lane 1, 1:6000; lane 2, 1:9000; lane 3, 1:12,000; lane 4, 1:15,000; and lane 5, 1:18,000. Antiserum specificity determined by preincubation with peptide or purified GST–Cyp519–201 fusion protein is as follows: lane 6, 5 μg; lane 7, 500 ng; lane 8, 50 ng; and lane 9, 5 ng. Numbers at left indicate molecular weight markers in kilodaltons.
(B) Immunoblot of 30 μg of total protein from different Arabidopsis organs detected with 1:6000 dilutions of anti-Cyp5 peptide antiserum and anti-GNOM Sec7 antiserum (Steinmann et al., 1999). The 19-kD Cyp5 and 165-kD GNOM bands are shown.
(C) Localization of Cyp5 protein by subcellular fractionation. Immunoblot of protein extracts from cell suspensions expressing the Golgi apparatus marker Myc-sialyl transferase (ST2-11) (Wee et al., 1998) subjected to differential centrifugation. S10, supernatant of 10,000g centrifugation; S100, cytosolic supernatant; P100, microsomal membrane pellet of 100,000g centrifugation. Protein gel blots were probed with an anti-Myc antibody (A14; Santa Cruz Biotechnologies, Santa Cruz, CA; 1:1000) for control of membrane integrity, as well as anti-GNOM Sec7 and anti-Cyp5 peptide antisera. The 19-kD Cyp5, 165-kD GNOM (arrow), and 46- and 48-kD ST2-11 (Myc) bands are shown with short and long exposure of Cyp5 detection.
(D) Localization of Cyp5 in cell suspension cultures. Cyp5 protein in extracts from suspension cells (Culture cells) and cell culture supernatant (Culture medium) on day 5 after passage was detected by immunoblotting with anti-Cyp5 peptide antiserum. The 19-kD Cyp5 band is shown. Culture supernatant was concentrated by ammonium sulphate precipitation (Saito et al., 1999).