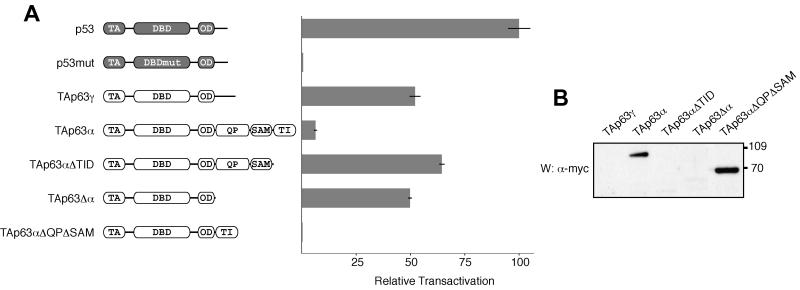
FIG. 1.
The last 71 residues of the α C terminus of p63 contain an inhibitory domain. (A) The relative transactivation levels of different p63 isoforms and deletion mutants were compared with those of the wild type and a DNA-binding-incompetent mutant of p53, as described in Materials and Methods. All values are scaled relative to the transactivation of wild-type p53, which was set to 100%. On the left the domain structure of the individual transcripts is indicated: TA (transactivation domain), DBD (DNA-binding domain), OD (oligomerization domain), QP (glutamine- and proline-rich domain), SAM (sterile alpha motif domain), TI (transactivation-inhibitory domain). TAp63αΔTID, residues 1 to 570; TAp63Δα, residues 1 to 397; TAp63αΔQPΔSAM, residues 1 to 397 and 568 to 641. (B) The lysates of p63-transfected cells used to measure relative transactivation (above) were resolved on an SDS-4 to 12% PAGE gradient gel, transferred to a PVDF membrane, and Western blotted with c-Myc-specific antibody 9E10. The bands that appear correspond to the expected molecular weights. The three species with high transactivation potential, TAp63γ, TAp63αΔTID, and TAp63Δα, are not visible, while the inactive species, TAp63α and TAp63αΔQPΔSAM, are abundant. Numbers at right are molecular weights in thousands.