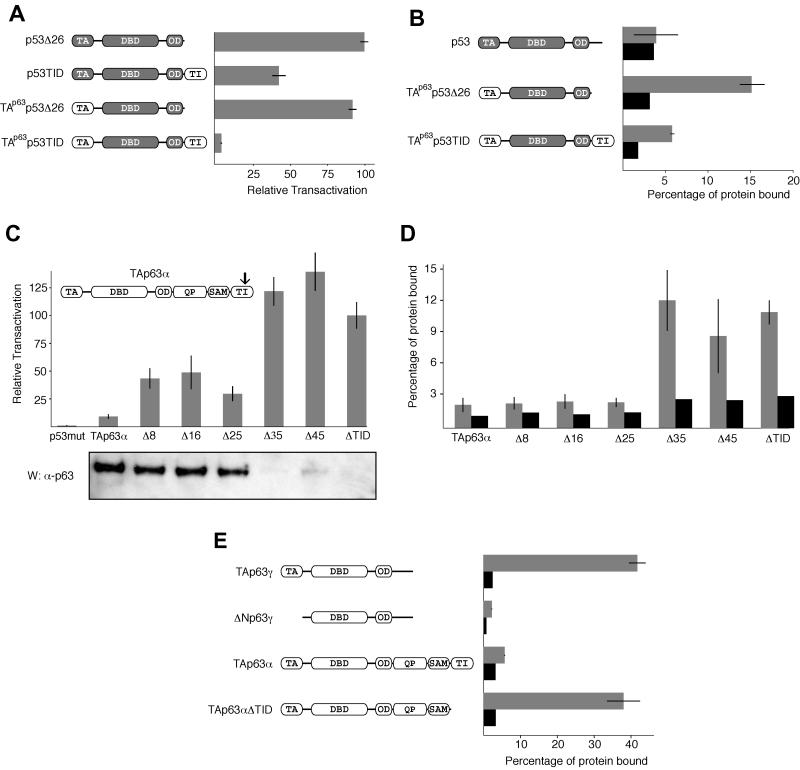
FIG. 6.
The TI domain is a specific inhibitor of the p63 TA domain. (A) Relative transactivation of different chimeras composed of swapped domains of p63 and p53. p53Δ26, residues 1 to 361 of p53; p53TID, residues 1 to 361 of p53 and 568 to 641 of p63; TAp63p53Δ26, residues 1 to 127 of p63 and 92 to 361 of p53; TAp63p53TID, residues 1 to 127 of p63, 92 to 361 of p53, and 568 to 641 of p63. Shaded segments represent p53 domains, and nonshaded segments represent p63 domains. (B) GST pull-down assay on different p53/p63 chimeras. The gray bars show the percentages of protein bound to beads loaded with a GST-TID fusion protein, and the black bars show the amounts of protein bound to beads loaded with GST only. (C) The relative transactivation levels of TAp63α with various C-terminal truncations were compared with that for a DNA-binding-incompetent mutant of p53. All values are scaled relative to the transactivation of TAp63αΔTID, which was set to 100%. TAp63α mutants lacking the last 8 (Δ8), 16 (Δ16), or 25 (Δ25) amino acids show a moderate increase in activity relative to full-length TAp63α. Δ35, Δ45, and ΔTID (missing the last 71 residues) have high activity. The arrow indicates the approximate location of the truncations. The lysates of transfected cells used to measure relative transactivation were resolved on an SDS-4 to 12% PAGE gradient gel, transferred to a PVDF membrane, and Western blotted with the anti-p63 4A4 antibody. The bands correspond to the expected molecular weights, gradually decreasing from left to right. (D) Results of GST-TID pull-down assays with C-terminally truncated forms of TAp63α. GST-TID fails to bind TAp63α presumably because the intramolecular association is more favorable than the same association in trans. Removal of the last 35 or more residues leads to a sudden and dramatic improvement in binding most likely caused by a disruption of the intramolecular TI-TA domain interaction. (E) GST pull-down assay with a truncated TI domain. The affinities of different p63 isoforms for a truncated form of the TI domain lacking the last 25 amino acids (GST-TIDΔ25) were measured. The results are very similar to the results obtained with GST fused to the full-length TI domain.