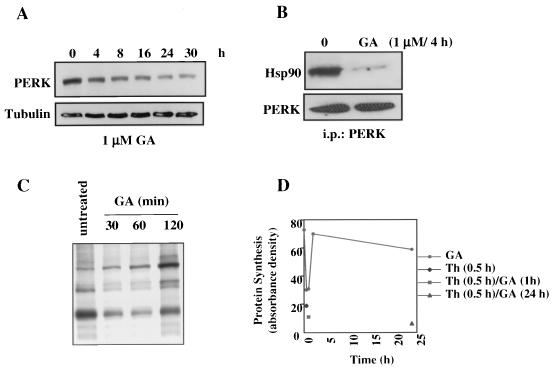
FIG. 6.
The ER transmembrane kinase PERK associates with HSP90 but is functionally less sensitive to GA than is IRE1. (A) AR42J cells were treated with GA (1 μM) for the times indicated, cells were lysed, and proteins were resolved by SDS- 10% PAGE and transferred to nitrocellulose membranes. Western analysis of PERK steady-state levels was performed with an anti-PERK antibody. Tubulin was blotted to demonstrate equal loading of protein. (B) AR42J cells were left untreated or treated with GA (1 μM for 4 h) and then lysed, and 400 μg of soluble proteins were immunoprecipitated with 2 μg of anti-PERK antibody. Antibody complexes were bound on protein A-Sepharose beads, washed, eluted, and resolved by SDS-10% PAGE. After transfer to a nitrocellulose membrane, Western analysis of coprecipitated HSP90 was performed. The immunoprecipitates were also blotted for PERK to demonstrate its equivalent pulldown in the presence and absence of GA. (C) AR42J cells were treated with GA (1 μM) for the indicated times. The cells were then pulse-labeled with 100 μCi of [35S]methionine/cysteine/ml for 30 min and lysed. Equal amounts of total soluble proteins were analyzed by SDS-PAGE and autoradiography. (D) AR42J cells were treated with thapsigargin (Th; 0.5 μM) for 0.5 h (filled diamond), GA (1 μM) for 0.5, 1, 2, or 24 h (filled circles) or with a combination of GA and Th (filled square and filled triangle; GA was added for the indicated time, followed by Th for 30 min). Cells were then pulse-labeled and processed as for panel C. Equal amounts of total cell proteins were analyzed by SDS-PAGE and autoradiography.