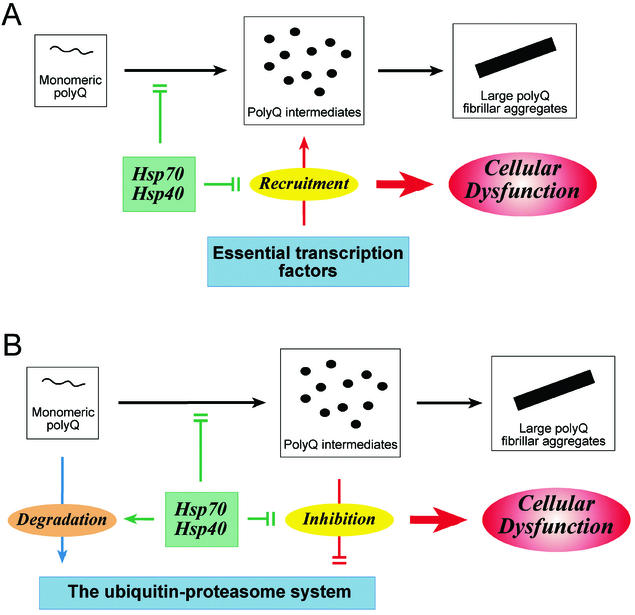
Figure 2.
Two models for polyQ-mediated toxicity and its suppression by the Hsp70/Hsp40 chaperone system. (A) Transcriptional dysregulation. Intermediates of polyQ aggregation recruit essential transcription factors, thereby inhibiting their transcriptional activities and causing cellular dysfunction. The Hsp70/Hsp40 chaperone system prevents this recruitment and thus mitigates polyQ toxicity. (B) Inhibition of the ubiquitin–proteasome system. PolyQ aggregation intermediates trap the 19S regulatory complex (and other components of the degradative machinery), resulting in a partial inhibition of proteasome-dependent proteolysis and eventually cellular dysfunction. The Hsp70/Hsp40 chaperone system may prevent or reduce this effect by stabilizing polyQ protein in a soluble, degradation-competent state and shielding aggregates.