Abstract
Background
Benign prostatic hyperplasia affects older men. This systematic review determined efficacy and adverse effects of finasteride.
Review methods
PubMed, the Cochrane Library, reference lists of reports, and reviews were searched for randomised, double-blind trials of finasteride in benign prostatic hyperplasia. Outcomes included symptom score, urinary flow rate, prostate volume, discontinuation, and adverse effects. Relative risk and NNT or NNH were calculated for dichotomous data. Sensitivity analyses assessed influences of baseline symptom severity, initial prostate volume, a dominating trial, and previous interventions.
Results
Three trials had active controls and 19 had placebo. In placebo-controlled trials, 8820 patients received finasteride 5 mg and 5909 placebo over 3–48 months. Over 48 months finasteride produced greater improvements in total symptom score, maximum urinary flow rate, and prostate volume. Significantly more sexual dysfunction, impotence, ejaculation disorder and decreased libido occurred with finasteride at 12 months; the NNH for any sexual dysfunction at 12 months was 14. Significantly fewer men treated with finasteride experienced acute retention or had surgery at 24 or 48 months than with placebo; at 12 months the NNT was 49 (31 to 112) to avoid one acute urinary retention and 31 (21 to 61) to avoid one surgery. Sensitivity analyses showed benefit with finasteride 5 mg to be constant irrespective of the initial prostate volume.
Conclusions
Information from many patients in studies of high quality showed beneficial effects of finasteride in terms of symptoms, flow rate and prostate volume. More utility would result if patient centred outcomes were reported in dichotomous form.
Background
Benign prostatic hyperplasia (BPH) properly describes the histological basis of a diagnosis of prostatic enlargement leading to bladder outflow obstruction that gives rise to symptoms of lower urinary tract obstruction [1]. Symptoms of benign prostatic enlargement occur commonly in older men. In an unselected population of Scottish men prevalence rates increased from 615 per thousand in the fifth decade to 890 per thousand in the eighth decade [2]. With time symptoms generally get worse. Over five years symptom scores in men with predominantly mild symptoms deteriorate by two points [3]. About 18% of men with initially mild symptoms will develop symptoms that are moderate over five years, with about three per thousand becoming severe [3] though severe symptoms can ameliorate with time. Over five years perhaps only 3% of men with initially mild symptoms might seek treatment [3].
Outcomes chosen in clinical trials of treatments for benign prostatic hyperplasia include not only symptom scores, but maximum urinary flow rate, postvoid volume and prostate volume, as well as clinical outcomes such as acute urinary retention or progression to surgery [4-7]. Changes in these outcomes may occur even without active treatment, with reductions (improvements) in symptoms scores and increases (improvements) in maximum urinary flow rate [8]. For this reason accurate evaluation of potential benefit of interventions for symptomatic BPH require controlled trials of at least two years duration [8].
For many alternative therapies such studies are lacking [4]. Studies of alpha-blockers are generally less than two years [5,9,6]. Studies of interventions like transurethral microwave thermotherapy may have longer follow up of between three and seven years, but the bulk of the information is from nonblinded, uncontrolled studies [10], and in surgical studies men generally have higher initial symptom scores and lower maximum urinary flow rates than is seen in medical interventions.
For finasteride some systematic reviews and meta-analyses already exist [5,11]. A significant proportion of randomised trials of finasteride have lasted one or two years, and at least one large study continued beyond two years [12]. Our aim in this systematic review and meta-analysis was to examine results for the standard dose of 5 mg finasteride according to duration of treatment so that men and their professional advisers would know what to expect, and when, both with and without treatment.
Materials and methods
Searching
PubMed (to April 2001) and the Cochrane Library (Issue 2, 2001) were searched to identify full journal publications of randomised, double blind, placebo and active controlled trials of finasteride in the treatment of benign prostatic hyperplasia. Free text search terms used included 'finasteride', 'proscar', 'clinical trial', and 'benign prostatic hyperplasia'. Systematic reviews of finasteride [5,11] were examined, as was a list of systematic reviews in benign prostatic hyperplasia (additional file 1) for possible references and reference lists of all obtained articles were checked to identify additional trials. Abstracts were not sought. Merck, Sharp and Dohme Ltd, UK, were asked for references of any published randomised trials for finasteride in the context of benign prostatic hyperplasia. Unpublished studies were not sought.
It was anticipated that patient information from major trials may have been published more than once, in part or in full, as information became available from longer use of finasteride. For each trial, the study that provided the fullest amount of information was included in the systematic review and any duplicated information was excluded. Duplicate studies were checked to ensure that relevant information for a particular outcome described in an excluded study was not missing from the included trial. A number of trials had an open-label extension in which men received finasteride only; no open-label information was analysed because the pre-hoc decision was to analyse only information from trials that were both randomised and double blind.
Each report which could possibly be described as a randomised controlled trial was read independently by both authors and scored using a commonly-used, three item, 1–5 score, quality scale [13]. Disagreements were discussed and consensus achieved. The maximum score of an included study was 5 and the minimum score was 2.
Outcomes were abstracted after discussion with a panel of two urologists and three general practitioners with an interest in urology to discuss outcomes likely to be of professional or patient interest. Information extracted from the double blind trials included: (i) number of men on finasteride and placebo, (ii) symptom score (total, obstructive, bother), (iii) prostate volume, (iv) urinary flow rate (maximum, mean), (v) discontinuation (total discontinuations, discontinuations because of lack of efficacy, discontinuations because of adverse effects), (vi) information on adverse effects including episodes of acute urinary retention and prostate surgery, (vii) prostate specific antigen (PSA), (viii) residual volume and (ix) total voided volume. Absolute values and/or mean or median values (with dispersion) were extracted. When provided, this information was extracted for the following time-points regardless of whether it was an intermediate or final assessment: baseline and 3, 6, 12, 18, 24, 36 and 48 months of treatment. The type of symptom score used was noted.
Analysis of data
Not all men in a trial may have had each outcome assessed (e.g. prostate volume), or men may have discontinued. For efficacy analyses, therefore, the number of men for which information was available at a particular point in time was used if this differed from the number randomised; because results were rarely dichotomous, an intention-to-treat analysis was impossible for any beneficial outcome. For analysis of adverse effects and discontinuations, the number of men randomised to treatment was used to provide an intention-to-treat analysis.
When possible, patient information from different studies was pooled. The objective was to enter any continuous data in Review Manager (RevMan version 4.01; Update Software, Oxford), to calculate weighted mean difference from baseline for finasteride and for placebo, to generate standard deviations or 95% confidence intervals, and to determine statistical significance of differences between treatments at various time points. Weighted mean values (by group size) for continuous outcomes were calculated using Excel:mac 2001 on a Macintosh G4.
When dichotomous information was available, relative risk estimates, with 95% confidence intervals, were calculated using a fixed effects model [14]. A statistically significant difference between active treatment and placebo was assumed when the 95% confidence intervals (CI) of the relative risk did not include unity. Numbers-needed-to-treat, with 95% confidence intervals, were calculated [15]. The confidence interval of the NNT includes no benefit of one treatment over the other when the upper limit includes infinity. NNT is the reciprocal of the absolute risk reduction or increase; for instance, if 75 out of 100 men benefit with treatment and only 25 out of 100 benefit with placebo, the absolute risk increase is 0.75–0.25 = 0.5, and the NNT is 1/0.5 = 2.
Neither heterogeneity tests nor funnel plots were used since they lack the power to reliably detect statistical heterogeneity or publication bias [16-18]. Instead, pre-planned sensitivity analyses were conducted to detect possible variations in effect of study treatments in men with differing aetiology or baseline severity of their condition.
The four sensitivity analyses were:
1. Comparison of differing severity of symptoms at baseline – results of studies which included men with moderate or severe symptoms at baseline were compared with those which also included men with mild symptoms at baseline.
2. Comparison of differing prostate volume at baseline – results of studies which included men with small prostates (mean less than 40 cm3) were compared with those which included men with large (mean 40 cm3 or greater) prostates at baseline. The reason for this is that there is some evidence that finasteride is more effective in men with prostate volumes greater than 40 cm3 [11].
3. Comparison of the results of other trials with those from a dominating single, large, four year, double blind trial (PLESS) [19].
4. Comparison of the results in men without prior interventional treatments and those who may have had a previous stent or balloon dilatation.
Analyses based on fewer than 500 men are not presented in this report, because the results would not be robust [20].
Results
Trials available for analysis
One hundred and one reports were identified as potential randomised trials of finasteride in the treatment of benign prostatic hyperplasia. Several of these were duplicate publications, with or without reference to previous publications. Eighty studies were excluded and reasons are given in additional file 2. Twenty-two double blind randomised trials from 21 reports were included in the systematic review [19,21-40]. Nineteen trials were placebo controlled, and three compared finasteride with another active treatment. Full details of the included studies, with description of men included, study duration, outcomes, and results are shown in additional file 3, and all the baseline characteristics of study groups in additional file 4.
Finasteride 5 mg daily was assessed in all included trials, though some also included finasteride 1 mg or 10 mg. One trial pooled information from 17 men with finasteride 1 mg and 15 men with finasteride 5 mg [37]; this information was included in the analyses described below.
In placebo controlled trials, 8820 men received finasteride 5 mg given once daily and 5909 men received placebo. Information was available for men followed up for three months in two trials (74 men), six months in nine (366), 12 months in six (6364), 18 months in one (55), 24 months in four (4286), and for 48 months in one (3082). Three trials used an active control; 903 men received finasteride 5 mg once daily, 553 permixon (serenoa repens), and 358 alfluzosin 10 mg daily. Men were followed up for six months in two studies (1870 men) and for 12 months in one trial (489 men). Trials of active comparisons were not followed up further because the comparators were all different and numbers of men were small.
Men included in the trials had a clinical diagnosis of benign prostatic hyperplasia, mostly based on symptoms and urine flow rates. For symptoms, for instance, the American Urological Association symptom scoring scale uses seven questions which can be scored from 0 (no problem) to 5 (severe problem); the scale can be from 0 to 35, split into mild (0–7 points), moderate (8–19 points) or severe (20–35 points) disease. At baseline, symptoms were moderate to severe in 17 trials, and in two studies men with mild symptoms were included. General exclusions in the trials were men with the suggestion of prostate cancer, urinary tract infection, previous prostate surgery, haematuria, or those who required catheterisation for acute urinary retention.
A summary of the included trials, their size at randomisation, quality score, duration, intention-to-treat (by number randomised) or per protocol analysis, and the statistical significance at longest double-blind estimate is given in Table 1. Size varied from 36 to 3040 randomised men; nine trials randomised at least 500 men and four at least 2000. The largest trials were of at least 12 months duration. Most of the larger trials demonstrated a significant superiority of finasteride 5 mg over placebo at the p < 0.01 level for symptom score, maximum urinary flow rate and prostate volume; the notable exception was the VA Cooperative study for symptom scores and maximum urinary flow rate because the inclusion criteria for this study included men with small prostates [31].
Table 1.
Summary of results in the individual randomised, double blind, placebo controlled trials.
| Author | Number of patients | Quality score | Duration (months) | ITT or PP | Symptom score | Max urinary flow rate (mL/s) | Prostate volume (cm3) |
| McConnell et al, 1998 PLESS | 3040 | 4 | 48 | PP | ++ | ++ | ++ |
| Marbergher et al, 1998 PROWESS | 2902 | 5 | 24 | PP | ++ | ++ | ++ |
| Byrnes et al, 1995 | 2417 | 3 | 12 | ITT | + | No data | |
| Tenover et al, 1997 | 2112 | 3 | 12 | PP | ++ | No data | |
| Andersen et al, 1995 | 707 | 3 | 24 | PP | ++ | ++ | ++ |
| Lepor et al, 1996 VA cooperative study. | 615 | 4 | 12 | PP | - | - | ++ |
| Nickel et al, 1996 PROSPECT | 613 | 5 | 24 | ITT | ++ | ++ | ++ |
| Gormley et al, 1992 North American study | 598 | 3 | 12 | ITT | ++ | ++ | ++ |
| Finasteride study group, 1993 International study | 501 | 3 | 12 | PP | ++ | + | ++ |
| Beisland et al, 1992 | 182 | 3 | 6 | ITT | + | + | ++ |
| Abrams et al, 1999 | 121 | 3 | 12 | PP | - | + | ++ |
| Kirby et al, 1992 | 50 | 4 | 3 | PP | ++ | ++ | - |
| Yu et al, 1995 | 50 | 3 | 6 | PP | + | + | + |
| Marks et al, 1997 | 41 | 4 | 6 | PP | - | - | ++ |
| Tammela & Kontturi, 1993 | 36 | 3 | 6 | ITT | No data | - | ++ |
| Studies with finasteride plus additional interventions | |||||||
| Lukkarinen et al, 1999 | 61 | 3 | 24 | PP | - | - | ++ |
| Isotalo et al, 2001 | 55 | 4 | 18 | ITT | - | + | ++ |
+ p < 0.05 ++ p < 0.01 – No significant difference between finasteride and placebo ITT Intention to treat analysis – all randomised patients PP Per protocol analysis – may have included men who discontinued treatment
Trial reports usually provided dispersion measures (standard deviation, interquartile ranges) at baseline for measures like symptom score, urine flow rate and prostate volume. In almost no case did they provide standard deviations or 95% confidence intervals for intermediate and/or final assessment for these outcome data. Consequently statistical significance of differences between finasteride and placebo could not be assessed for continuous measures.
Symptom scores
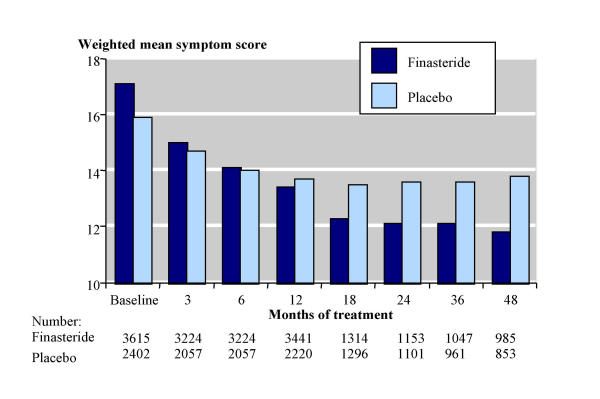
Three different scoring systems were used. In placebo-controlled trials, the Boyarsky scale (0–54) was used in two studies, modified Boyarsky scale (0–36) in nine, and the American Urological Association (AUA; 0–35, similar to the current International Prostate Symptom Score, IPSS) in eight; higher scores show worse symptoms in all three scales. Symptom severity was recorded at baseline and at various stages throughout the study, though not all studies reported this information for all time points, including baseline. Figure 1 shows the weighted mean total symptom scores at different time points with the American Urological Association scale which was used for most men, and the results for all scales are in additional file 5. Total symptom scores were similar at baseline with finasteride (17) and placebo (16). They then fell (improved), and by 12 months there was a greater reduction in symptom score with finasteride (by 3.7 points) than with placebo (by 2.3 points), and maintained for 24 to 48 months. Scores continued to decrease with finasteride up to 48 months, whereas they started to increase with placebo after 18 months.
Figure 1.
Total symptom scores using the American Urological Association scale (0–35).
Maximum urinary flow rate
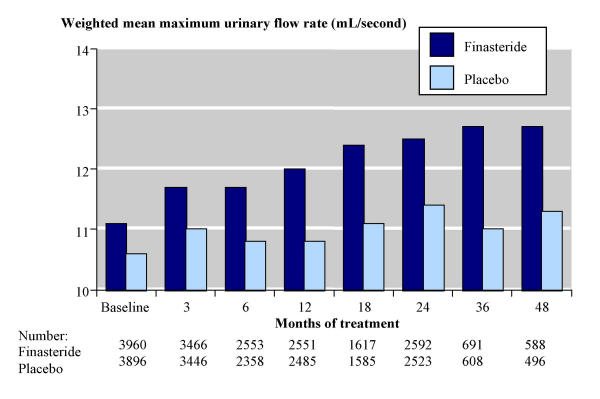
Figure 2 shows the results for maximum urinary flow rate. At baseline the weighted mean maximum urinary flow rate was 11.2 mL/s with finasteride (3960 men) and 10.5 mL/s with placebo (3893 men). This increased (improved) with time on treatment and by 24 months weighted mean urinary flow rates were 12.5 mL/s with finasteride (2592 men) and 11.3 mL/s with placebo (2523 men).
Figure 2.
Maximum urinary flow rate over time.
Prostate volume
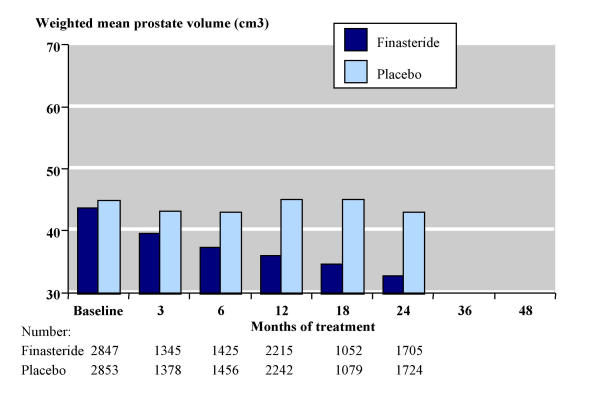
Figure 3 shows the results for prostate volume, which declined by 25% over 24 months with finasteride compared with a 4% decline with placebo. At baseline the weighted mean prostate volume was 43.7 cm3 with finasteride (2847 men) and 44.8 cm3 with placebo (2853 men). This decreased (improved) with duration of finasteride treatment, but generally increased (worsened) with placebo. By 24 months prostate volume was 32.7 cm3 with finasteride (1705 men) and 43.0 cm3 with placebo (1724 men). Prostate volumes of about 30 cm3 or less are generally assumed to be normal in older men and not associated with prostatic hyperplasia [41].
Figure 3.
Prostate volume over time.
Sensitivity analyses
Symptom severity at baseline
Information was pooled from studies that included men with mild plus moderate symptoms at baseline, and was compared with pooled information from studies including only men with at least moderate symptoms at baseline. Only two trials included men with mild symptoms, with fewer than 500 per group. There was insufficient information to determine whether symptom severity at baseline affected treatment efficacy in terms of prostate volume or urinary flow rate.
Prostate volume at baseline
The sensitivity of results to prostate volume was investigated in two ways. In the first, results were pooled according to whether mean prostate volume of the finasteride-treated group at baseline was less than 40 cm3 or 40 cm3 or greater. Prostate volume and maximum urinary flow rate over time were determined for each data set (additional file 6). By 24 months finasteride achieved a similar proportional reduction in prostate volume with larger (79% of placebo) and smaller (75% of placebo) prostates. There was limited data for maximum urinary flow rate in men with smaller prostates, but there was no evidence that the increased flow rate at 24 months was higher with men with larger (110% of placebo) or smaller (108% of placebo) prostates.
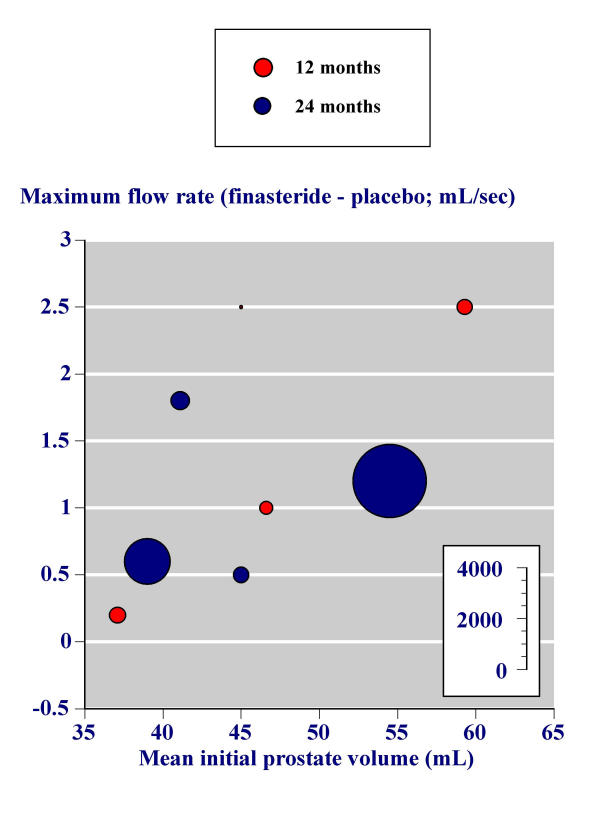
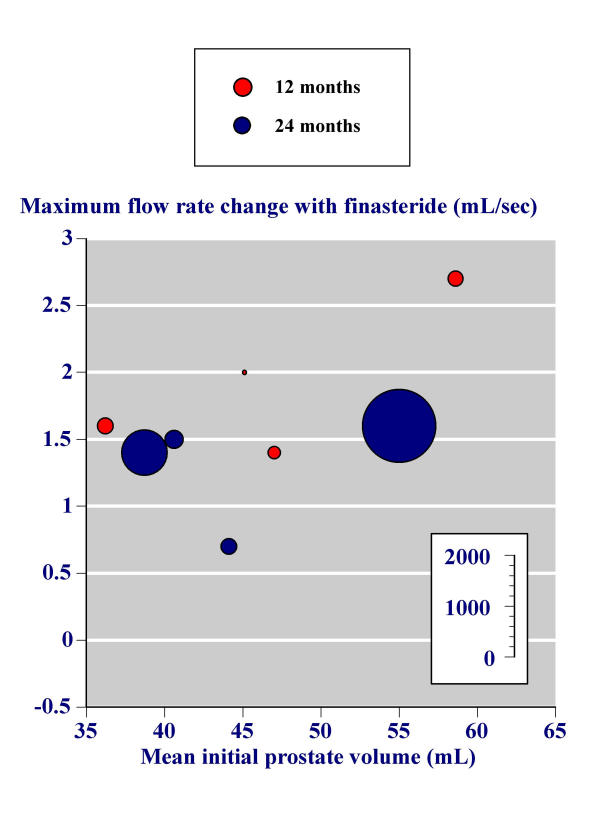
In the second, the effect of initial prostate volume on the success of finasteride in increasing maximum urinary flow rate in the individual trials was investigated graphically. Figure 4 replicates the analysis of Boyle and colleagues [11] using all trials with more than 100 men and with 12 or 24 month outcomes. In this analysis the difference between the change in maximum urinary flow rate between baseline and study end with placebo is subtracted from the change in maximum urinary flow rate between baseline and study end with finasteride; what is plotted is the difference between two differences. Higher baseline prostate volume was associated with larger increases in maximum urinary flow rate for finasteride minus placebo.
Figure 4.
Effect of initial prostate volume – Replication of the analysis by Boyle and colleagues.
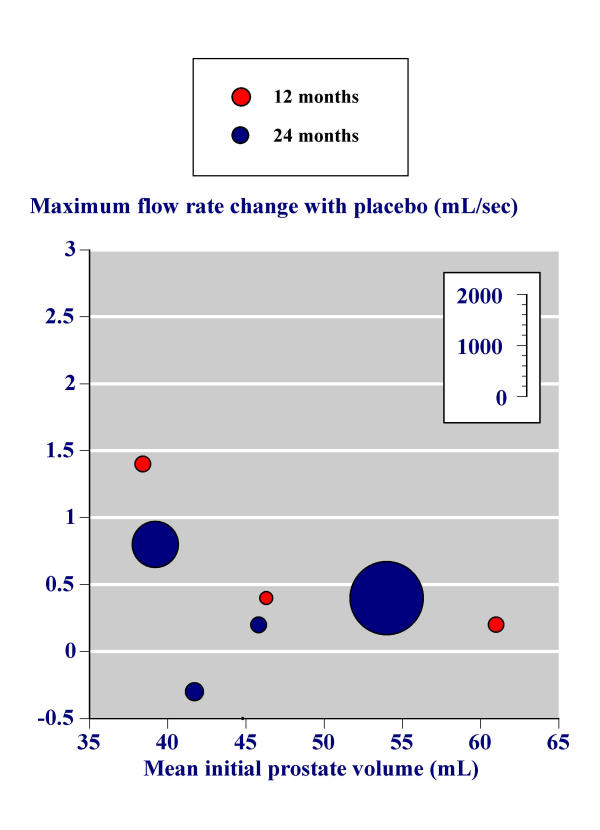
These analyses were performed for the change in maximum urinary flow rate between baseline and study end with finasteride (Figure 5) and the change in maximum urinary flow rate between baseline and study end with placebo (Figure 6). The benefit with finasteride 5 mg was constant irrespective of the initial prostate volume (Figure 5). For placebo, men with higher prostate volumes had little overall change in maximum urinary flow rate, while men with lower prostate volumes had maximum urinary flow rates that increased.
Figure 5.
Effect of initial prostate volume – finasteride.
Figure 6.
Effect of initial prostate volume – placebo.
Influence of PLESS
Table 2 shows the results of analyses using the PLESS trial [19] compared with results of other trials (excluding PLESS). Not all men in the PLESS trial had prostate volumes measured. In those in whom it was, the 24 month analysis showed approximately a 10 cm3 reduction in prostate volume (18% reduction from baseline of 55 cm3 with finasteride) for PLESS and other trials, compared with a 4 cm3 (8%) increase in volume with placebo. Maximum urinary flow rates at 24 months rose with finasteride to the same extent (by about 2 mL per second) in the PLESS trial with 1125 men and in the studies with 2991 men given finasteride.
Table 2.
Comparison of other studies with information from PLESS
| A. Prostate volume (cm3) | |||||
| PLESS | Other studies (without PLESS) | ||||
| Finasteride | Placebo | Finasteride | Placebo | ||
| Baseline | Number | 157 | 155 | 2719 | 2696 |
| Value | 55.0 | 54.0 | 43.1 | 44.2 | |
| 24 months | Number | 130 | 119 | 2146 | 2140 |
| Value | 45.1 | 58.3 | 31.5 | 41.5 | |
| Mean change from baseline at 24 mths | -9.9 | 4.3 | -10.6 | 3.3 | |
| B. Maximum urinary flow rate (mL/s) | |||||
| PLESS | Other studies (without PLESS) | ||||
| Finasteride | Placebo | Finasteride | Placebo | ||
| Baseline | Number | 1125 | 1127 | 2991 | 2923 |
| Value | 11.0 | 11.0 | 10.5 | 10.3 | |
| 24 months | Number | 786 | 720 | 1806 | 1803 |
| Value | 12.6 | 11.4 | 12.4 | 11.3 | |
| Mean change from baseline at 24 mths | 1.6 | 0.4 | 1.9 | 1.0 | |
Number of patients at baseline, or remained in the studies at 24 months
Previous interventions
Only two trials [29,32] included men who had prior interventions like balloon dilatation, or catheterisation with a stent implant. Together these two studies had only 59 men on finasteride and 60 on placebo. Sensible comparisons were not possible.
Discontinuation
Discontinuation was well reported in the trials.
Significantly fewer men discontinued with finasteride than with placebo at 12 months or 48 months (Table 3A). After 12 months, all cause discontinuation rates were 13% (553/4098 men) with finasteride and 17% (299/1764) with placebo; number-needed-to-treat to prevent one discontinuation was 29 (18 to 71). After 48 months, discontinuation rates were 34% (524/1524 men) with finasteride and 42% (633/1516) with placebo; NNH to prevent one discontinuation was 13 (9.0 to 27).
Table 3.
Discontinuations in double blind, placebo controlled trials of 3–48 months duration Significantly fewer discontinuations with finasteride than with placebo.
| A. Total number of patients who discontinued | |||||||
| Number of studies | Time point (months) | Discontinued with finasteride 5 mg | Discontinued with placebo | Relative risk (95% CI) | NNT (95% CI) | ||
| Number | Percent | Number | Percent | ||||
| 1 | 3 | No data | |||||
| 5 | 12 | 553/4098 | 13 | 299/1764 | 17 | 0.8 (0.7 to 0.9) | 29 (18 to 71) |
| 4 | 24 | 467/2146 | 22 | 507/2140 | 24 | 0.9 (0.8 to 1.03) | n/c |
| 1 | 48 | 524/1524 | 34 | 633/1516 | 42 | 0.8 (0.7 to 0.9) | 13 (9 to 27) |
| B. Discontinuations because of lack of efficacy | |||||||
| Number of studies | Time point (months) | Discontinued with finasteride 5 mg | Discontinued with placebo | Relative risk (95% CI) | NNT (95% CI) | ||
| Number | Percent | Number | Percent | ||||
| 1 | 3 | No data | |||||
| 4 | 12 | 116/3788 | 3 | 46/1459 | 3 | 0.9 (0.7 to 1.4) | n/c |
| 4 | 24 | 81/2146 | 4 | 107/2140 | 5 | 0.8 (0.6 to 1.0) | n/c |
| 1 | 48 | 99/1524 | 6 | 104/1516 | 7 | 0.9 (0.7 to 1.2) | n/c |
| C. Discontinuations because of adverse effects | |||||||
| Number of studies | Time point (months) | Discontinued with finasteride 5 mg | Discontinued with placebo | Relative risk (95% CI) | NNT (95% CI) | ||
| Number | Percent | Number | Percent | ||||
| 1 | 3 | No data | |||||
| 5 | 12 | 251/4098 | 6 | 89/1764 | 5 | 1.2 (0.9 to 1.5) | n/c |
| 1 | 18 | No data | |||||
| 4 | 24 | 178/2146 | 8 | 210/2140 | 14 | 0.8 (0.7 to 1.02) | n/c |
| 1 | 48 | 176/1524 | 12 | 166/1516 | 11 | 1.0 (0.9 to 1.3) | n/c |
NB: Number-needed-to-treat to prevent one discontinuation with finasteride compared with placebo
There was no significant difference between groups at any time point for discontinuation because of lack of efficacy (Table 3B), with 24 month discontinuations because of lack of efficacy at 4% with finasteride and 5% with placebo. There was no significant difference between groups at any time point for discontinuation because of adverse effects (Table 3C), with 24 month discontinuations because of adverse effects at 8% with finasteride and 14% with placebo.
Specific adverse effects
The most commonly reported adverse effects were impotence, decreased libido and ejaculation disorder. Definitions of these adverse effects were generally not provided in the trials. Since cumulative adverse effect information was not available after year one in some studies, analyses were conducted for different time points up to one year for most adverse effects. Numbers-needed-to-harm are shown in Table 4 for analyses for which there were adequate data. Serious adverse effects occurred at similar frequencies with finasteride (12%; 437/3557 men) as with placebo (13%; 150/1175 men).
Table 4.
Adverse effects reported in double blind, placebo controlled trials of 3–48 months duration Significantly fewer adverse effects with finasteride than with placebo.
| Adverse effect | Number of studies | Time point (months) | Harmed with finasteride 5 mg | Harmed with placebo | Relative risk (95% CI) | NNT (95% CI) | ||
| Number | Percent | Number | Percent | |||||
| Serious adverse effects | 2 | 12 | 437/3557 | 12 | 150/1175 | 13 | 1.0 (0.8 to 1.1) | n/c |
| Any sexual dysfunction | 1 | 12 | 239/1736 | 14 | 38/579 | 7 | 2.1 (1.5 to 2.9) | 14 (10 to 22) |
| Decreased libido | 5 | 12 | 269/5688 | 5 | 86/3296 | 3 | 2.0 (1.6 to 2.5) | 47 (35 to 74) |
| Impotence | 6 | 12 | 439/5394 | 8 | 117/3551 | 3 | 2.2 (1.8 to 2.7) | 24 (20 to 31) |
| Ejaculation disorder | 5 | 12 | 137/5688 | 2 | 19/3296 | 0.6 | 3.6 (2.2 to 6.0) | 55 (43 to 74) |
| Acute urinary retention | 3 | 12 | 17/3803 | 0.4 | 9/1430 | 0.6 | 0.8 (0.4 to 1.9) | n/c |
| 1 | 24 | 24/1450 | 2 | 54/1452 | 4 | 0.5 (0.3 to 0.7) | 49 (31 to 112) | |
| 1 | 48 | 42/1542 | 3 | 99/1516 | 7 | 0.4 (0.3 to 0.6) | 26 (19 to 44) | |
| BPH related surgery | 5 | 12 | 54/4410 | 1 | 24/2035 | 1 | 1.1 (0.7 to 1.7) | n/c |
| 2 | 24 | 85/1760 | 5 | 142/1755 | 8 | 0.6 (0.5 to 0.8) | 31 (21 to 61) | |
| 1 | 48 | 69/1542 | 4 | 152/1516 | 10 | 0.5 (0.3 to 0.6) | 18 14 to 27) | |
| Prostate cancer | 2 | 12 | 9/3557 | 0.3 | 5/1175 | 0.4 | 0.6 (0.2 to 0.8) | n/c |
| 1 | 24 | 3/310 | 1 | 6/303 | 2 | 0.5 (0.1 to 1.9) | n/c | |
| 1 | 48 | 76/1524 | 0.5 | 76/1516 | 0.5 | 1.0 (0.7 to 1.4) | n/c | |
n/c Not calculable NB: For acute urinary retention, prostate surgery, and prostate cancer the NNT represents the number-needed-to-treat to prevent one episode of acute urinary retention or prostate surgery
Significantly more men reported any sexual dysfunction, decreased libido, impotence, or ejaculation disorder with finasteride than with placebo at 12 months of treatment. Incidence rates ranged between 2–14% with finasteride and 0.6–7% with placebo, and NNHs for particular adverse events ranged between 14 and 55 (Table 4). This means, for instance, that for every 24 men treated with 5 mg finasteride for 12 months impotence would occur in one in whom it would not have occurred with placebo.
Avoiding AUR and surgery
Significantly fewer men suffered acute urinary retention or surgery related to benign prostatic hyperplasia after 24 months (Table 4), though much of this information was derived from the PLESS trial. Acute urinary retention and surgery related to benign prostatic hypertrophy occurred in less than 1% of men over the first 12 months of treatment. By 24 months their occurrence was significantly lower with finasteride than with placebo. The NNTs for avoiding acute urinary retention were 49 (31 to 112) over 24 months and 26 (19 to 44) over 48 months. The NNTs for avoiding prostate related surgery were 31 (21 to 61) over 24 months and 18 (14 to 27) over 48 months. This means, for instance, that for every 31 men treated with 5 mg finasteride for 24 months prostate surgery would be avoided in one in whom it would have occurred with placebo.
It was not possible to calculate results for a combined end point of avoiding acute urinary retention or surgery.
There was no statistically significant difference in the incidence of prostate cancer with finasteride compared with placebo. Rates were under 0.5% at 12 months, and in one four year study [19] rates were 1–2% at 24 months and 0.5% at 48 months.
Discussion
Clinical trials set out to determine whether an intervention is better than no treatment (placebo) or another treatment. Their conduct is governed by rules of evidence, and in the main they will need to be randomised and double blind to minimize bias, have a valid design, measure useful clinical outcomes over a sensible period, and be of sufficient size to minimize the effects of random chance or to have a reasonable expectation of seeing a difference if one exists. How clinical trials are designed will often depend on what happens without treatment. For instance, after surgery some patients do not get pain [42], and so trials of analgesics recruit only patients who have moderate or severe pain in the first place [43]. For men with mild or moderate symptoms of BPH long duration placebo-controlled trials are essential; while the natural history is for symptoms to worsen with time [8], lifestyle changes or tolerance mean that in some men improvement occurs spontaneously [3].
Clinical practice is different because the choice is whether to use an intervention or not, or which of several interventions to use. Placebo is not an option, though watchful waiting may be a sensible decision for many men [44]. The choice made will be a product of how much symptoms interfere with aspects of daily living, professional advice, and individual preference. Knowledge of the natural history [45] will be part of the decision-making, together with knowledge of what to expect from treatment.
This review sought to explore whether randomised trials of finasteride 5 mg (the standard licensed dose in the UK) could be used to provide an adequate estimate of what to expect from treatment in terms of benefits (symptoms, flow rates) and harm (acute retention, progression to surgery, and adverse event discontinuation). The 19 trials available comparing finasteride 5 mg with placebo all had quality scores of 3 out of 5 or higher, indication that they were likely to be free of major bias [46,47]. Ten trials, with the bulk of the patients randomised, were of at least 12 month's duration and included more than 500 patients. Almost all showed finasteride 5 mg to be significantly better than placebo for each of three efficacy outcomes (symptoms, maximum urinary flow rate and prostate volume), and mostly at a significance level of 1%. The evidence is that finasteride is effective.
The question is, what can a man expect from treatment with finasteride?
At baseline the individual studies recruited men with mean symptom scores of 8 to 19 points, usually described as moderate. Treatment with finasteride 5 mg for 12 months resulted in a 4 point decrease and for 24 months in a 5 point decrease, so that using the American Urological Association score mean values fell from 17 points at baseline to 12 points. The range of scores for moderate symptoms is 8–19 points, so on average men could expect symptoms to reduce from just under severe to just over mild. Mean urinary flow rates would rise from a mean baseline value of 11.2 mL/s with finasteride 5 mg to 12.5 mL/s after 24 months of treatment; a flow rate of 12 mL/sec is seen by some as a threshold for BPH [41]. This would be accompanied by a reduction in prostate volume by a mean of 8 cm3 to an average of about 33 cm3, and though this would not be apparent to the patient it comes close to the one threshold of normality for prostate volume [41]. Quality of life was looked at in several trials, but the challenge is to find an acceptable disease-related instrument that adds to current disease measures [48].
Out of every 100 men with moderate symptoms treated with finasteride 5 mg over two years, 34 would discontinue treatment for any reason. For six men it would be because of lack of treatment benefit, for 12 it would be because of adverse events. Four men out of 100 would become impotent, who would not have done with no treatment. Additional benefits would be that out of 100 men treated for two years two would avoid an episode of acute urinary retention and three would avoid prostate-related surgery.
The adverse events seen with finasteride have to be seen in context. Firstly they appear to be reversible on stopping treatment, and we found no evidence of rare, major and irreversible adverse events, important for treatment taken for the rest of a man's life. Discontinuations due to adverse events were the same in finasteride and placebo-treated men, and at 8% with finasteride after 24 months about the same as seen in alpha-blockers in trials generally of much shorter duration [49]. The sexual adverse events have to be seen against the natural history of reducing sexual performance with age. For instance, sexual dysfunction in men is common [50] and increases with age [51]; men aged 55 years without erectile dysfunction consider intercourse frequency as being less than adequate [52]. The men in the finasteride trials had an average age of about 65 years.
It is clear that increasing prostate volume is the cause of clinical benign prostatic hyperplasia [45]. Prostate volume is not usually measurable in general practice because the required ultrasound equipment is not available. However, PSA acts as a surrogate measure for prostate volume, and as a predictor for increased risk of acute urinary retention [53]. It should be noted, however, that finasteride decreases PSA values by a factor of about two, important to remember if prostate cancer were suspected [54].
The relationship between efficacy of finasteride and prostate volume [55] has been important in making decisions about treatment [11]. Based on an individual patient analysis of six trials Boyle and colleagues showed that while men treated with finasteride 5 mg all had the same average increase in flow rate over one year, statistical significance from placebo occurred only with prostate volumes above 40 cm3. Improvements in symptom score tended to be higher in men with larger prostates. Analysing mean data from all trials with more than 100 men per treatment group confirms this (Figures 4,5,6). With finasteride 5 mg a mean improvement in maximum flow rate of about 1.5 mL/sec can be expected (Figure 5) irrespective of initial prostate size (Figure 5).
Dependence of treatment efficacy on prostate size is likely be related to mechanism of action, as no such relationship has yet been demonstrated with the alpha-adrenergic receptor antagonist terazosin [9]. In terms of choosing who should be treated, the largest and longest randomised trial of finasteride suggest that men with higher symptom scores and a baseline PSA of above 1.4 ng/mL will benefit most [12]. This is confirmed by a combined analysis of all men given placebo in finasteride trials [53]. Spontaneous acute urinary retention over two or four years was low when the initial PSA was less than 1.3 ng/mL, but occurred in 2% of men with initial PSA between 1.4 and 3.2 ng/mL and was over 5% in men with a PSA above 3.3 ng/mL. PSA alone was as effective as more complicated algorithms at predicting acute retention [53].
Limitations of the review lie mainly in the way that efficacy trials in BPH are conducted and reported. For outcomes like adverse events, discontinuations or events like acute retention or prostate surgery, the number of men with the event is reported; with the number of men randomised an intention-to-treat analysis becomes possible, and pooling of data using standard methods is possible. Not all trials reported any or all of these outcomes, but they were reported in most of the larger trials in a reasonably consistent manner.
Efficacy outcomes were another matter. There are three problems. First is the relevance of the outcomes themselves. For instance, maximum urinary flow rate is a useful measure of urinary outlet obstruction, though multiple measures are probably more useful than a single measure [56]. A maximum flow rate of less than 15 mL/sec is generally considered suboptimal, though some now use a flow rate of 12 mL/sec. So a useful outcome might be the number of men at each time point who achieve a maximum urinary flow rate of 15 mL/sec (or 12 mL/sec [41], or whatever is deemed clinically useful). Trials report only mean flow rates, and, with some exceptions at baseline, without any dispersion data in the form of standard deviations or confidence intervals. So, despite many trials involving nearly 14,000 men with BPH, it is not possible to say what proportion can achieve any given maximum flow rate with finasteride or placebo, and when. Reporting of symptom scores is similar, so we cannot say what proportion of men with initially moderate symptoms would have symptoms reverting to mild with finasteride or placebo, or when.
Conclusions
Reporting problems in BPH treatments is not limited to finasteride trials, and is common with all treatments [5], including alpha-blockers [9], as well as other remedies [4]. What would be useful would be some clear indications of patient-centred outcomes, as has been done for migraine [57]. Reporting these outcomes in dichotomous form (success or failure) would greatly aid understanding of the benefits for men, and for healthcare systems.
Competing interests
RAM has been a consultant for MSD, though not in the area of benign prostatic hyperplasia. RAM & JE have received lecture fees from pharmaceutical companies, thought not in the area of benign prostatic hyperplasia. The authors have received research support from charities, government and industry sources at various times, but no such support was received for this work. Neither author has any direct stock holding in any pharmaceutical company. The terms of the financial support from MSD included freedom for authors to reach their own conclusions, and an absolute right to publish the results of their research, irrespective of any conclusions reached. MSD did have the right to view the final manuscript before publication, and did so; MSD made no substantive comments, and the manuscript was not changed as a result.
Authors' contributions
JE performed the searches and extracted data which was checked by RAM. Both authors read the papers, and contributed equally to analysis, and writing and reviewing the paper.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Systematic reviews and meta-analyses on benign prostatic hyperplasia.
Excluded studies.
Details of included studies.
Baseline characteristics of men in the included studies.
Absolute total symptom score.
Effect of large or small prostate volume at baseline on response.
Acknowledgments
Acknowledgements
A panel of urologists and general practitioners (Ian Eardley, Mike Kirby, John Pillinger, Robert Schuster-Bruce and Mark Speakman) provided invaluable advice and comments. We are grateful for their comments on the design of the study, and on an early version of the manuscript. Their efforts made it better, but any errors are ours. Financial support was provided by an unconditional educational grant from Merck Sharp and Dohme Ltd, UK. Other support came from the Oxford Pain Relief Trust and Pain Relief funds.
Contributor Information
Jayne E Edwards, Email: jayne.edwards@pru.ox.ac.uk.
R Andrew Moore, Email: andrew.moore@pru.ox.ac.uk.
References
- Nordling J, Hald T. BPH versus LUTS: reflections on lower urinary tract symptoms in search of a definition of clinical benign prostatic hyperplasia. European Urology Update Series. 1997;6:54–60. [Google Scholar]
- Simpson RJ. Benign prostatic hyperplasia. An overview of epidemiology and treatment. Primary Care in the New NHS. 2001. pp. 184–186.
- Lee AJ, Garraway WM, Simpson RJ, et al. The natural history of untreated lower urinary tract symptoms in middle-aged and elderly men over a period of five years. Eur Urol. 1998;34:325–332. doi: 10.1159/000019749. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Ishani A, Rutks I, et al. A systematic review of Cernilton for the treatment of benign prostatic hyperplasia. BJU International. 1999;85:836–841. doi: 10.1046/j.1464-410x.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- Clifford GM, Farmer RDT. Medical therapy for benign prostatic hyperplasia: a review of the literature. Eur Urol. 2000;38:2–19. doi: 10.1159/000020246. [DOI] [PubMed] [Google Scholar]
- McNeill SA, Hargreave TB, Geffriaud-Ricouard C, et al. Postvoid residual urine in patients with urinary tract symptoms suggestive of benign prostatic hyperplasia: pooled analysis of eleven controlled studies with alfuzosin. Urology. 2001;57:459–465. doi: 10.1016/S0090-4295(00)01021-9. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Roehrborn CG. Benign prostatic hyperplasia. BMJ. 2001;323:1042–1046. doi: 10.1136/bmj.323.7320.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC. Placebo therapy of benign prostatic hyperplasia: a 25-month study. British Journal of Urology. 1998;81:383–387. doi: 10.1046/j.1464-410x.1998.00554.x. [DOI] [PubMed] [Google Scholar]
- Boyle P, Robertson C, Manski R, et al. Meta-analysis of randomised trials of terazosin in the treatment of benign prostatic hyperplasia. Urology. 2001;58:717–722. doi: 10.1016/S0090-4295(01)01344-9. [DOI] [PubMed] [Google Scholar]
- Ramsey EW, Dahlstrand C. Durability of results obtained with transurethral microwave thermotherapy in the treatment of men with symptomatic benign prostatic hyperplasia. Journal of Endourology. 2000;14:671–675. doi: 10.1089/end.2000.14.671. [DOI] [PubMed] [Google Scholar]
- Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomised clinical trials. Urology. 1996;48:398–405. doi: 10.1016/S0090-4295(96)00353-6. [DOI] [PubMed] [Google Scholar]
- Kaplan S, Garvin D, Gilhooly P, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia-related outcomes and long-term response to finasteride. Urology. 2000;56:610–616. doi: 10.1016/S0090-4295(00)00724-X. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. London British Medical Journal. 1995. pp. 50–63. [DOI] [PMC free article] [PubMed]
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. British Medical Journal. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- Tang JL, Liu Y. Misleading funnel plot for detection of bias in meta-analysis. Journal of Clinical Epidemiology. 2000;53:477–484. doi: 10.1016/S0895-4356(99)00204-8. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. The New England Journal of Medicine. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- Moore RA, Gavaghan D, Tramèr MR, et al. Size is everything – large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–216. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Abrams P, Schäfer W, Tammela TLJ. Improvement of pressure flow parameters with finasteride is greater in men with large prostates. The Journal of Urology. 1999;161:1513–1517. doi: 10.1097/00005392-199905000-00026. [DOI] [PubMed] [Google Scholar]
- Andersen JT, Ekman P, Wolf H, et al. Can finasteride reverse the progress of benign prostatic hyperplasia? A two-year placebo-controlled study. Urology. 1995;46:631–637. doi: 10.1016/S0090-4295(99)80291-X. [DOI] [PubMed] [Google Scholar]
- Beisland HO, Binkowitz B, Brekkan E. Scandinavian clinical study of finasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 1992;22:271–277. doi: 10.1159/000474771. [DOI] [PubMed] [Google Scholar]
- Byrnes CA, Morton AS, Liss CL, et al. Efficacy, tolerabiliy, and effect on health-related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: a community-based study. Clinical Therapeutics. 1995;17:956–969. doi: 10.1016/0149-2918(95)80073-5. [DOI] [PubMed] [Google Scholar]
- Carraro JC, Raynaud JP, Koch G. Comparison of phtyotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: a randomised international study of 1,098 patients. The Prostate. 1996;29:231–240. doi: 10.1002/(SICI)1097-0045(199610)29:4<231::AID-PROS4>3.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Debruyne FMJ, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. Eur Urol. 1998;34:169–175. doi: 10.1159/000019706. [DOI] [PubMed] [Google Scholar]
- Finasteride Study Group Finasteride (MK-906) in the treament of benign prostatic hyperplasia. The Prostate. 1993;22:291–299. doi: 10.1002/pros.2990220403. [DOI] [PubMed] [Google Scholar]
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effects of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- Isotalo T, Talja M, Hellström P, et al. A double-blind, randomised, placebo-controlled pilot study to investigate the effects of finasteride combined with a biodegradable self-reinforced poly L-lactic acid spiral stent in patients with urinary retention caused by bladder outlet obstruction from benign prostatic hyperplasia. BJU International. 2001;88:30–34. doi: 10.1046/j.1464-410x.2001.02250.x. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Bryan J, Eardley I, et al. Finasteride in the treatment of benign prostatic hyperplasia. A urodynamic evaluation. British Journal of Urology. 1992;70:65–72. doi: 10.1111/j.1464-410x.1992.tb15666.x. [DOI] [PubMed] [Google Scholar]
- Lepor H, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- Lukkarinen O, Lehtonen T, Talja M, et al. Finasteride following balloon dilatation of the prostate. A double-blind, placebo-controlled, multicenter study. Annales Chirurgiae et Gynaecologiae. 1999;88:299–303. [PubMed] [Google Scholar]
- Marberger MJ. Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. Urology. 1998;51 doi: 10.1016/s0090-4295(98)00094-6. [DOI] [PubMed] [Google Scholar]
- Marks LS, Partin AW, Gormley GJ, et al. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. The Journal of Urology. 1997;187:2171–2178. doi: 10.1097/00005392-199706000-00040. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Fradet Y, Boake RC, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomised controlled trial (the PROSPECT Study). Can Med Assoc J. 1996;155:1251–1259. [PMC free article] [PubMed] [Google Scholar]
- Sökeland J, Albrecht J. Kombination aus Sabal- und Urticaextrakt vs. Finasterid bei BPH (Stad. I bis II nach Alken). Urologe (A) 1997;36:327–333. doi: 10.1007/s001200050106. [DOI] [PubMed] [Google Scholar]
- Stoner E, Finasteride Study Group The clinical effects of a 5a-reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Journal of Urology. 1992;147:1298–1302. doi: 10.1016/s0022-5347(17)37547-x. [DOI] [PubMed] [Google Scholar]
- Tammela TLJ, Kontturi MJ. Urodynamic effects of finasteride in the treatment of bladder outlet obstruction due to benign prostatic hyperplasia. The Journal of Urology. 1993;149:342–344. doi: 10.1016/s0022-5347(17)36077-9. [DOI] [PubMed] [Google Scholar]
- Tenover JL, Pagano GA, Morton AS, et al. Efficacy and tolerability of finasteride in symptomatic benign prostatic hyperplasia: a primary care study. Clin Ther. 1997;19:243–258. doi: 10.1016/S0149-2918(97)80113-0. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Chiu TY, Lai MK. Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomised double-blind controlled trial. J Formos Med Assoc. 1995;94:37–41. [PubMed] [Google Scholar]
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. The Journal of Urology. 1997;158:481–487. doi: 10.1097/00005392-199708000-00040. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Bullingham RE, Moore RA, et al. Some patients don't need analgesics after surgery. Journal of the Royal Society of Medicine. 1982;75:705–708. [PMC free article] [PubMed] [Google Scholar]
- McQuay HJ, Moore RA. An evidence-based resource for pain relief. Oxford Oxford university Press. 1998.
- Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethal surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med. 1995;332:75–79. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001;58:5–16. doi: 10.1016/S0090-4295(01)01298-5. [DOI] [PubMed] [Google Scholar]
- Khan KS, Daya S, Jadad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Arch Intern Med. 1996;156:661–666. doi: 10.1001/archinte.156.6.661. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? [see comments]. Lancet. 1998;352:609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Batista-Miranda JE, Diez MD, Bertran PA, et al. Quality-of-life assessment in patients with benign prostatic hyperplasia: effects of various interventions. Pharmacoeconomics. 2001;19:1079–1090. doi: 10.2165/00019053-200119110-00002. [DOI] [PubMed] [Google Scholar]
- Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract. 1998;15:519–524. doi: 10.1093/fampra/15.6.519. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- Dinsmore WW, Hodges M, Hargreaves C, et al. Sildenafil citrate (Viagra) in erectile dysfunction: near normalization in men with broad-spectrum erectile dysfunction compared with age-matched healthy control subjects. Urology. 1999;53:800–805. doi: 10.1016/S0090-4295(98)00586-X. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Malice M, Cook TJ, et al. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPH: a comprehensive analysis of the pooled placebo groups of several large clinical trials. Urology. 2001;58:210–216. doi: 10.1016/S0090-4295(01)01155-4. [DOI] [PubMed] [Google Scholar]
- Guess HA, Gormley J, Stoner E, et al. The effect of finasteride on prostate specific antigen: review of available data. Merck Research Laboratories Review Article. 1996;155:3–9. [PubMed] [Google Scholar]
- Tammela TL, Schafer W, Barrett DM, et al. Repeated pressure-flow studies in the evaluation of bladder outlet obstruction due to benign prostatic enlargement. Finasteride Urodynamics Study Group. Neurourol Urodyn. 1999;18:17–24. doi: 10.1002/(SICI)1520-6777(1999)18:1<17::AID-NAU4>3.3.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Reynard JM, Peters TJ, Lim C, et al. The value of multiple free-flow studies in men with lower urinary tract symptoms. Br J Urol. 1996;77:813–818. doi: 10.1046/j.1464-410X.1996.00097.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache. 1999;39:S20–S26. doi: 10.1111/j.1526-4610.1999.00006.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Systematic reviews and meta-analyses on benign prostatic hyperplasia.
Excluded studies.
Details of included studies.
Baseline characteristics of men in the included studies.
Absolute total symptom score.
Effect of large or small prostate volume at baseline on response.