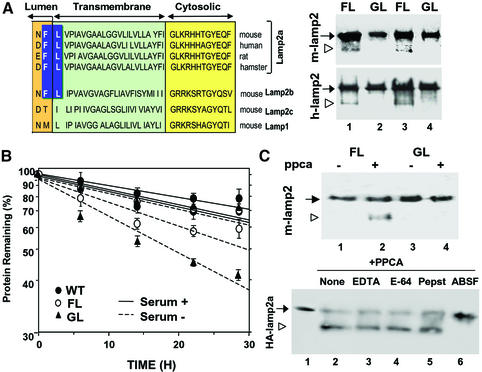
Fig. 7. Cleavage site of PPCA in lamp2a. (A) Left: amino acid sequences of the C-terminal regions of lamp2a from different species and of other lamp2 isoforms and lamp1 from mouse. The proposed cleavage site for PPCA in lamp2a is highlighted. Right: immunoblot analysis for HA of lysosomal membranes isolated from two different clones of HEK293 (mouse; upper panel) and NIH 3T3 cells (human; lower panel) stably transfected with wild-type (FL) and mutated (GL) HA-lamp2a. The arrowhead indicates the truncated form of lamp2 lacking the cytosolic/transmembrane region. (B) Total rates of protein degradation in the presence and absence of serum in non-transfected HEK293 cells (WT) and in the transfected HEK293 clones described in (A). Values are expressed as the percentage of initially radiolabeled proteins remaining at each time and are means ± SE of three different experiments. (C) Top: immunoblot analysis with an anti-HA antibody of purified wild-type (FL) and mutated (GL) mouse HA-lamp2a incubated in an acidic pH buffer alone, or with PPCA. Bottom: wild-type HA-lamp2a was incubated as in the top panel without additions (None) or in the presence of 2 mM EDTA, 10 µM l-trans-epoxysuccinyl-leucylamide-(4-guanido)-butane (E-64), 2 µM pepstatin A (Pepst) or 10 µM ABSF. The open arrowhead indicates the truncated form of lamp2a.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.