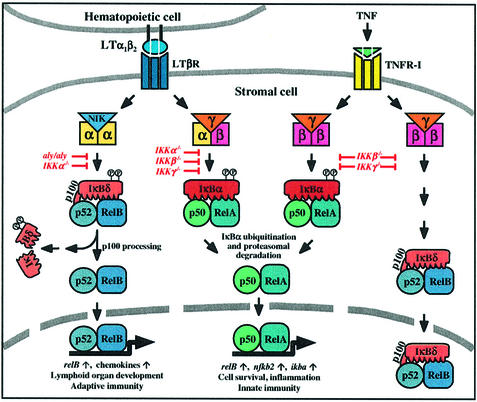
Fig. 8. Model of LTβR- and TNFR-I-mediated activation of NF-κB. Signaling through TNFR-I activates the NF-κB pathway involving the β and γ subunits of the IKK complex. Nuclear translocation and DNA binding of p50–RelA heterodimers is accomplished through IκBα phosphorylation and ubiquitin-dependent degradation. This pathway also upregulates the expression of RelB and the p52 precursor, p100, resulting in the specific inhibition of RelB by the C-terminal IκBδ domain of p100. Membrane-bound LTα1β2 heterodimers, on the other hand, activate LTβR, which also results in the induction of RelA complexes, but the requirement for IKK subunits differs from the TNFR pathway. Importantly, LTβR signaling triggers the degradation of p100 in a NIK- and IKKα-dependent manner. As a consequence, p52–RelB heterodimers accumulate in the nucleus and regulate genes that are crucial for the normal development of lymphoid organs.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.