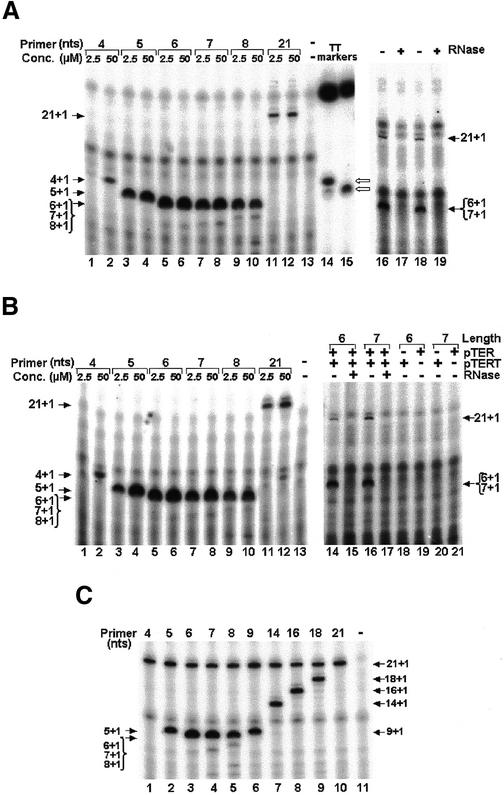
Figure 3.
Determination of the minimal length required for a primer aligned in the middle of the TER template region, to be extended by a single nucleotide. Primers of the series shown in Figure 1A were extended with native Tetrahymena telomerase (A), or with a telomerase core that was reconstituted by co-expression of the Tetrahymena TERT and TER in a rabbit reticulocyte lysate (B), or with an immunopurified reconstituted core telomerase (C). In the assays shown in (A) and (B) (lanes 1–12), the primers at the two indicated concentrations were incubated with the telomerase in the presence of 32P-labeled dGTP alone. In (C) (lanes 1–10), each reaction was similarly performed with equimolar concentrations (2.5 µM) of the 21mer oligo and the indicated primer. Lane 13 in (A) and (B) and 11 in (C) show control assays performed with no primer. Lanes 14 and 15, respectively, in (A) show markers produced by extension of the 4mer and the 5mer oligos with terminal transferase. The marker bands are designated with open arrows. The strong bands at the top of these lanes are non-specific bands that were often observed in the terminal transferase reaction mixtures. Samples of the products were directly loaded and electrophoresed in a denaturing gel without being purified and ethanol precipitated. The arrows point at single bands that contained oligos, which were extended by a single 32P-labeled dGMP residue. Note that the 6mer, 7mer and 8mer products were not resolved in the gel. In the control assays shown in (A), the reactions were performed with 2.5 µM of primers and with telomerase that was preincubated with (lanes 17 and 19), or without RNase (lanes 16 and 18). Similar control assays are also shown in (B) (lanes 14–17). In other control assays shown in (B), the reactions were performed with reticulocyte lysates that produced only TERT (lanes 18 and 20), or only TER (lanes 19 and 21).