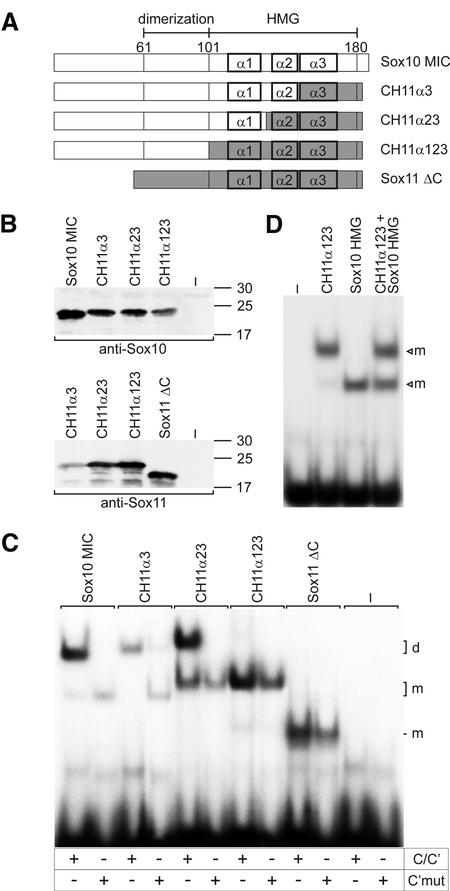
Figure 2.
Mapping of regions important for cooperative Sox10 binding within the Sox10 HMG domain. (A) Schematic representation of chimeric proteins generated by combining regions from the MIC variant of Sox10 and a C-terminally truncated form of Sox11 (Sox11ΔC). α1, α2 and α3 mark the three α-helices contained within the HMG domain of Sox proteins. Sox10-derived sequences are depicted as open boxes, Sox11-derived sequences as gray boxes. (B) Expression of all chimeras was verified by western blots of extracts from transfected COS cells with polyclonal antisera against Sox10 and Sox11 as indicated. Numbers indicate size of molecular weight markers in kDa. (C) Electrophoretic mobility shift assays with nuclear extracts from transfected COS cells expressing the proteins shown in (A). Oligonucleotides C/C′ and C′mut [in which C is mutated so that cooperative binding is lost, see Peirano and Wegner (12)] were used as probes as indicated below the lanes. (D) Electrophoretic mobility shift assays with nuclear extracts from transfected COS cells expressing the chimeric protein CH11α123 and the isolated Sox10 HMG domain alone or in combination as indicated above the lanes. –, extract from mock-transfected COS cells; m, bound monomer; d, bound dimer.