Abstract
Background
Results from numerous studies point convincingly to correlations between mutations at selected genes and phenotypic resistance to antimalarials in Plasmodium falciparum isolates. In order to move molecular assays for point mutations on resistance-related genes into the realm of applied tools for surveillance, we investigated a selection of P. falciparum isolates that were imported during the year 2001 into Europe to study the prevalence of resistance-associated point mutations at relevant codons. In particular, we tested for parasites which were developing resistance to antifolates and chloroquine. The screening results were used to map the prevalence of mutations and, thus, levels of potential drug resistance in endemic areas world-wide.
Results
337 isolates have been tested so far. Prevalence of mutations that are associated with resistance to chloroquine on the pfcrt and pfmdr genes of P. falciparum was demonstrated at high levels. However, the prevalence of mutations associated with resistance to antifolates at the DHFR and DHPS genes was unexpectedly low, rarely exceeding 60% in endemic areas.
Conclusions
Constant screening of imported isolates will enable TropNetEurop to establish a screening tool for emerging resistance in endemic areas.
Background
In an increasing number of countries where malaria is endemic, the effectiveness of routinely administered antimalarials like chloroquine and sulfadoxine/pyrimethamine is severely reduced because of the multi-drug resistance of Plasmodium falciparum. This poses a serious problem in terms of treatment and prophylaxis. Yet, mainly for financial reasons, chloroquine remains the first-line drug for treatment of malaria in these countries.
On the other hand, travelers from Europe and other industrialised countries, who have contracted Falciparum malaria, are commonly treated, on their return home, with highly effective antimalarials (usually mefloquine, halofantrine or quinine) that are frequently not freely available for routine treatment in endemic areas. Therefore, drug resistance is as yet not a frequent problem in the treatment of Falciparum malaria in hospitals of industrialized countries. Infected European travelers and immigrants carry a wide variety of P. falciparum strains from all endemic areas. Data and parasite material gained from this population, if properly used, can help to predict the development of drug resistance in endemic areas.
Point mutations at several codons of Plasmodium falciparum genes have been associated with emergence of drug resistance to commonly used antimalarial drugs. In particular, resistance to antifolates (e.g. pyrimethamine/sulfadoxine) and chloroquine depends on point mutations at the Dihydrofolate Reductase (DHFR) and Dihydropteroate Synthetase (DHPS) or Pfcrt and Pfmdr1 genes, respectively [1-3] . Several protocols for the detection of relevant mutations have been developed and have been evaluated with samples from endemic areas. Although a clear correlation between parasite genotype and in vivo outcome could not be documented in all field trials, the results show convincingly that a selected parasite population, if properly used, can help to correlations between point mutations and phenotypic resistance distinguish [4-9] . In order to move molecular assays for point mutations on resistance-related genes into the realm of applied tools for surveillance, we investigated a selection of P. falciparum isolates that were imported during the year 2001 into Europe to study the prevalence of resistance-associated point mutations at relevant codons. In particular, we tested isolates which were developing resistance to antifolates and chloroquine. The screening results were used for mapping prevalence of mutations and, thus, levels of potential drug resistance in endemic areas.
Methods
The study was established within the infrastructure of the European Network on Imported Infectious Disease Surveillance (TropNetEurop) which has been successfully providing surveillance data on imported malaria since 1999 [10] . The network covers approximately 12% of all imported malaria cases in Western and Central Europe. TropNetEurop is designed to effectively detect emerging infections of potential regional, national or global impact at their point of entry into the domestic population. Sentinel Surveillance reporting is carried out by participating sites using a standardized and computerized reporting system. Immediate transmission of anonymized patient and laboratory data to the central database assures timely detection of sentinel events. The comprehensive collection of data on notifiable and non-notifiable infectious diseases in travelers makes it possible to identify needs for further surveillance and investigation and provides the potential for future case-control studies by identification of specific risk factors. The primary objectives of TropNetEurop are a) to construct and maintain a collaborative research network of clinical sites in Europe dealing with imported infectious diseases and b) to establish and maintain a clinical network for effective sentinel surveillance of imported infectious diseases in Europe. Membership is self-selected by participating centers and monitored by the steering committee of the network. Although the organization of the network does not guarantee a representative data collection for Europe, most referral centers in Europe are represented. From the beginning, malaria has been one of the major targets within this network of 38 clinical sites throughout 15 European countries.
Detection of resistance-related point mutation followed established protocols. During standard malaria testing by thick and thin blood film, 10 μl of full blood from each patient were dotted on Whatman 3 MM® chromatography paper and air-dried at room temperature before initiation of treatment. DNA was prepared from the dried blood spots as previously described [11] . For detection of the single base change at codon 86 of Pfmdr1, a 330-basepair DNA fragment was amplified and followed by restriction-fragment-length-polymorphism (RFLP)-analysis [12,13] . A nested PCR protocol was used to identify the K76T in the Pfcrt gene [8,14] . After amplification of a 145 bp fragment around the codon 76, alleles carrying the K76 or T76 codon were discriminated by ApoI-restriction. For the detection of polymorphisms on the DHFR and DHPS genes, a previously described nested PCR method was used for all samples [6,15] . A volume of 4 μl of PCR product was incubated with mutation specific restriction enzymes according to the manufacturer's instructions (New England Biolabs, Beverly, MA, USA).
DHFR gene digest
To discriminate the three alternatives of codon 108 from each other, the 522 bp PCR product of the primer pair M3-F/ was digested with the following enzymes: AluI to detect the serine wild type (327 bp and 203 bp), BsrI to detect asparagine (309 bp and 190 bp) and BstNI to detect threonine (190 bp and 332 bp). The restriction site for Tsp509I was destroyed by an 51-asparagine to 51-isoleucine mutation (215 bp). Another PCR product was digested by XmnI to detect the 59-arginine mutation (162 bp).
DHPS gene digest
A 438 bp PCR product was digested by MnlI to detect 436-serine (283 bp), while digestion with MspAI identified the 436-alanine mutation (410 bp). Codon 437 was examined by digestion with AvaII to detect 437-glycine (402 bp) and MwoI to identify 437-alanine (387 bp). FokI served to distinguish between 540-lysine (404 bp) and 540-glutamic acid (320 bp). Another PCR product (436 bp) was digested by HindIII to identify the 436-phenylalanine mutation (410 bp). A PCR product of 161 bp was digested by BstUI to detect the 581-alanine wildtype (30 bp and 130 bp) and by BslI to discover 581-glycine mutations (30 bp and 130 bp). In the same way, the PCR product was digested by MwoI to identify 613-alanine (137 bp) and by BsaWI to detect the 613-serine/threonine mutation (130 bp). It was possible to differentiate 613-serine from 613-threonine by digestion with AgeI, which cuts in case of an 613-threonine mutation (128 bp).
All digestion products were separated by electrophoresis in an 1% SeaKem™ plus 1% NuSieve™ gel (FMC BioProducts, Rockland, ME, USA). As representative controls we used the established Plasmodium falciparum laboratory clones K1 and FCR3 as well as three own isolates that were gained during earlier studies in Uganda.
Results & Discussion
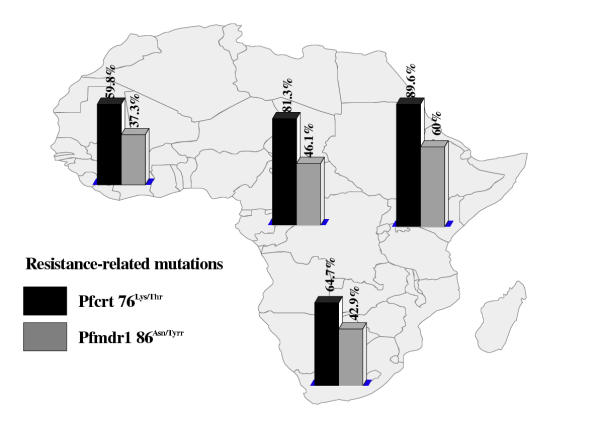
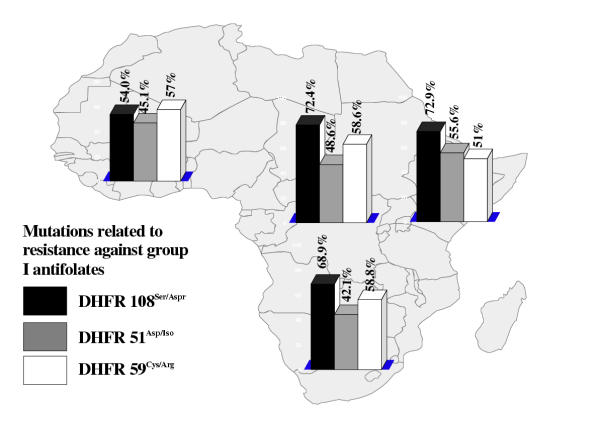
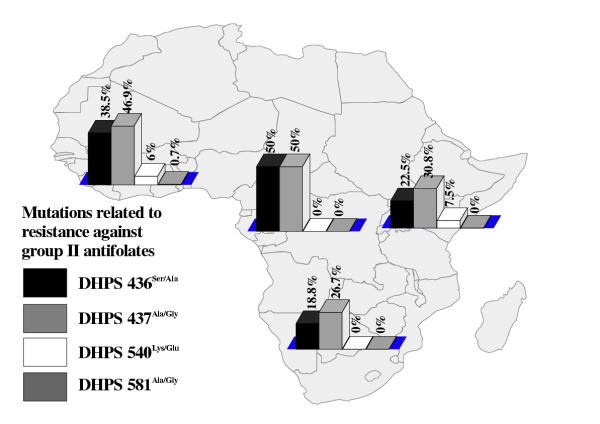
Altogether, 337 samples were screened (table 1). The majority (n = 313; 92.9%) of these were imported from sub-Saharan Africa. Out of the 24 samples (7.1%) from other endemic areas, 14 were from Indonesia. As the small numbers of isolates from Asia and the Americas were only of limited value for the determination of prevalence rates of point mutations, we omitted these areas from further evaluation. The total distribution of mutation-related point mutations among all isolates is shown in table 2. The mutation on Pfcrt76Lys/Thr has been identified as potentially crucial for developing resistance, while Pfmdr86Asn/Tyr appears to play a supporting role [16] . The majority of samples from sub-Saharan Africa showed at least former mutation (figure 1). However, a surprisingly high proportion of samples from areas that are generally assumed to have a very high level of drug resistance showed none of the mutations [17] . In particular, 40.2% of all samples from West Africa lacked the crucial prerequisite to develop phenotypical resistance to chloroquine (figure 1). A combination of both mutations was only present in 37.9% of the tested samples (data not shown). This pattern became even clearer when testing for mutations that are associated with resistance to antifolates. Here, DHFR108Ser/Asp has been identified as crucial mutation for resistance to group I antifolates, while DHFR51Asp/Iso and DHFR59Cys/Arg have a supporting function in enhancing the effect [1] . In similar fashion, DHPS437Ala/Gly is viewed as prerequisite for resistance to group II antifolates. Thus, P. falciparum has to gain mutations on both genes in order to develop resistance to combined antifolates, e.g. sulfadoxine/pyrimethamine. In the random population of isolates gained from returning European travelers, the distribution of the relevant mutations across sub-Saharan Africa was comparatively low (figures 2 and 3). The combination of triple mutations on DHFR (108Ser/Asp, 51Asp/Iso and 59Cys/Ar) was found in 39.2% of samples in West Africa, 30.8% in Central, 42.9% in East, and 33.3% in South Africa. The combination of DHPS437Ala/Gly and DHPS 540Lys/Glu, which is viewed as a necessary prerequisite for resistance to sulfonamides [9], was prevalent in 3.4% of the samples imported from West Africa, and 5.7% from East Africa, while no sample from either Central Africa, or from South Africa showed the combination. Thus, antifolates may still be effective in a comparatively high percentage of isolates from these areas.
Table 1.
Molecular surveillance of malaria drug resistance: geographical distribution of all isolates (n = 337)
| Region | Number | % |
| Central Africa | 39 | 11.6 |
| East Africa | 53 | 15.7 |
| Southern Africa | 20 | 5.9 |
| West Africa | 198 | 58.8 |
| Madagascar & African islands in the Indian Ocean | 3 | 0.9 |
| Central America | 1 | 0.3 |
| Caribbean | 2 | 0.6 |
| South America | 0 | 0 |
| West Asia | 0 | 0 |
| Indian Subcontinent | 5 | 1.5 |
| Indonesia | 14 | 4.2 |
| South East Asia | 2 | 0.6 |
| East Asia | 0 | 0 |
| Oceania | 0 | 0 |
| Total | 337 | 100 |
Table 2.
Total distribution of resistance-associated mutations and wild type among imported isolates of P. falciparum in Europe
| Resistance to | Gene locus | Mutation (%) | Wild type (%) | Total |
| Chloroquine | Pfcrt 76 | 204 (68.2%) | 95 (31.8%) | 299 |
| Pfmdr 86 | 85 (42.3%) | 116 (57.7%) | 201 | |
| Group I antifolates | DHFR 51 | 135 (44.3%) | 170 (55.7%) | 305 |
| DHFR 108 | 170 (58.4%) | 121 (41.6%) | 291 | |
| DHFR 59 | 156 (55.1%) | 127 (44.9%) | 283 | |
| Group II antifolates | DHPS 436 | 79 (33.3%) | 158 (66.7%) | 237 |
| DHPS 437 | 92 (40.4%) | 136 (59.6%) | 228 | |
| DHPS 540 | 12 (5.3%) | 216 (94.7%) | 228 | |
| DHPS 581 | 6 (2.3%) | 255 (97.7%) | 261 |
Group I antifolates: e.g. pyrimethamine. Group II antifolates: sulfonamides DHFR = dihydrofolate reductase DHPS = dihydropteroate synthetase
Figure 1.
Proportion of point mutations related to chloroquine resistance in different regions of sub-Saharan Africa (percentage showing proportion of mutation-positive samples from every geographical region)
Figure 2.
Proportion of point mutations related to resistance to group I antifolates in different regions of sub-Saharan Africa (percentage showing proportion of mutation-positive samples from every geographical region)
Figure 3.
Proportion of point mutations related to resistance to group II antifolates in different regions of sub-Saharan Africa (percentage showing proportion of mutation-positive samples from every geographical region)
Conclusions
This type of molecular surveillance has very little effect on treatment decisions for the individual travelers returning with Falciparum malaria from endemic regions. However, when used within a large clinical network, this method has an unsurpassed advantage. It has become possible to screen large numbers of isolates from malarious regions that are collected randomly from travelers and are transported back to Europe. This study shows that the continuous mapping of the patterns of resistance to crucial antimalarials can be performed. Data gained by fast and efficient molecular methods can be used as an early warning system for changes occurring in endemic areas, thus providing additional information that may be crucial for regional and international drug policy changes.
Acknowledgments
Acknowledgements
We wish to thank all site staff who have been invaluable locally in collecting material and data. This work was for European Network on Surveillance of Imported Infectious Diseases (TropNetEurop). TropNetEurop receives financial support from Dr. Democh Maurmeier Stiftung and Förderprogramm für Forschung und Lehre der Medizinischen Fakultät, both Ludwig-Maximilians-University, Munich, Germany. This help is gratefully acknowledged.
Contributor Information
Tomas Jelinek, Email: jelinek@lrz.uni-muenchen.de.
Gabriele Peyerl-Hoffmann, Email: peyerl@lrz.uni-muenchen.de.
Nikolai Mühlberger, Email: muehlberger@lrz.uni-muenchen.de.
Ole Wichmann, Email: ole.wichmann@lrz.uni-muenchen.de.
Michael Wilhelm, Email: michael.wilhelm@lrz.uni-muenchen.de.
Nadja Schmider, Email: nschmider@hotmail.com.
Martin P Grobusch, Email: martin.grobusch@uni-tuebingen.de.
Frank von Sonnenburg, Email: sonnenburg@lrz.uni-muenchen.de.
Joaquim Gascon, Email: jgascon@clinic.ub.es.
Hermann Laferl, Email: herman.laferl@kfj.magwien.gv.at.
Christoph Hatz, Email: hatz@keep.touch.ch.
Michael Alifrangis, Email: malif@biobase.dk.
Gerd Burchard, Email: gerd.buchard@charite.de.
Paul McWhinney, Email: P.McWhinney@pmail.net.
Marco Schulze, Email: mhschulze@yahoo.com.
Herwig Kollaritsch, Email: herwig.kollaritsch@univie.ac.at.
Saraiva da Cunha, Email: saraiva@huc.min-saude.pt.
Jiři Beřan, Email: beran@pmfhk.cz.
Peter Kern, Email: peter.kern@medizin.uni-ulm.de.
Ida Gjørup, Email: Ida.Gjorup@dadlnet.dk.
Juan Cuadros, Email: jcuadros@efd.net.
References
- Warhurst D. Drug resistance in Plasmodium falciparum malaria. Infection. 1999;27:S55–58. doi: 10.1007/BF02561674. [DOI] [PubMed] [Google Scholar]
- Ouellette M. Biochemical and molecular mechanisms of drug resistance in parasites. Trop Med Int Health. 2001;6:874–876. doi: 10.1046/j.1365-3156.2001.00777.x. [DOI] [PubMed] [Google Scholar]
- Wellems T, Plowe C. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- Curtis J, Duraisingh M, Trigg J, Mbwana H, Warhurst D, Curtis C. Direct evidence that asparagine at position 108 of the Plasmodium falciparum dihydrofolate reductase is involved in resistance to antifolate drugs in Tanzania. Trans R Soc Trop Med Hyg. 1996;90:678–680. doi: 10.1016/s0035-9203(96)90432-0. [DOI] [PubMed] [Google Scholar]
- Curtis J, Duraisingh M, Warhurst D. In Vivo Selection for a specific Genotype of Dihydropteroate Synthetase of Plasmodium falciparum by Pyrimethamine-Sulfadoxine but not Chlorproguanil-Dapsone Treatment. J Infect Dis. 1998;177:1429–1433. doi: 10.1086/517831. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Ronn AM, Lemnge MM, Curtis J, Mhina J, Duraisingh MT, Bygbjerg IC, Warhurst DC. Polymorphisms in the dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS) genes of Plasmodium falciparum and in vivo resistance to sulphadoxine/pyrimethamine in isolates from Tanzania. Trop Med Int Health. 1998;3:605–9. doi: 10.1046/j.1365-3156.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Kilian A, Curtis J, Duraisingh M, Kabagambe G, von Sonnenburg F, Warhurst D. Plasmodium falciparum: Selection of serine 108 of dihydrofolate reductase (DHFR) and alanine 581 of dihydropteroate synthetase (DHPS) during co-trimoxazole treatment of uncomplicated malaria in Ugandan children. Am J Trop Med Hyg. 1999;61:125–130. doi: 10.4269/ajtmh.1999.61.125. [DOI] [PubMed] [Google Scholar]
- Djimde A, Doumbo O, Cortese J, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su X, Nomura T, Fidock D, Wellems T, Plowe C. A molecular marker for chloroquine-resistant falciparum malaria. New Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Kublin J, Dzinjalamala F, Kamwendo D, Malkin E, Cortese J, Martino L, Mukadam R, Rogerson S, Lescano A, Molyneux M, Winstanley P, Chimpeni P, Taylor T, Plowe C. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Schulte C, Behrens R, Grobusch M, Coulaud J, Bisoffi Z, Matteelli A, Clerinx J, Corachán M, Puente S, Gjørup I, Harms G, Kollaritsch H, Kotlowski A, Björkmann A, Delmont J, Knobloch J, Nielsen L, Cuadros J, Hatz C, Beran J, Schmid M, Schulze M, Lopez-Velez R, Fleischer K, Kapaun A, McWhinney P, Kern P, Atougia J, Fry G, da Cunha S, Boecken G. Clinical and epidemiological characteristics among travellers and immigrants with imported Falciparum malaria in Europe: sentinel surveillance data from TropNetEurop. Clin Infect Dis. 2002;34:572–576. doi: 10.1086/338235. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Pröll S, Hess F, von Sonnenburg F, Kabagambe K, Löscher T, Kilian AHD. Geographical differences in the sensitivity of polymerase chain reaction for detection of P. falcipar um infection in Uganda. Am J Trop Med Hyg. 1996;55:647–651. doi: 10.4269/ajtmh.1996.55.647. [DOI] [PubMed] [Google Scholar]
- Duraisingh M, Drakeley C, Muller O, Bailey R, Snounou G, Targett G, Greenwood B, Warhurst D. Evidence for selection for the tyrosine-86 allele of the pfmdr 1 gene of Plasmodium falciparum by chloroquine and amodiaquine. Parasitology. 1997;114:205–211. doi: 10.1017/S0031182096008487. [DOI] [PubMed] [Google Scholar]
- Flüeck T, Jelinek T, Kilian A, Adagu I, Kabagambe G, von Sonnenburg F, Warhurst D. Correlation of in vivo-resistance to chloroquine and allelic polymorphisms in Plasmodium falciparum isolates from Uganda. Trop Med Int Health. 2000;5:174–178. doi: 10.1046/j.1365-3156.2000.00543.x. [DOI] [PubMed] [Google Scholar]
- Fidock D, Nomura T, Talley A, Cooper A, Dzekunov M, Ferdig T, Ursos M, Sidhu A, Naude B, Deitsch W, Su Z, Wootton C, Roepe D, Wellems E. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh M, Curtis J, Warhurst D. Plasmodium falciparum: Detection of Polymorphisms in the Dihydrofolate Reductase and Dihydropteroate Synthetase Genes by PCR and Restriction Digestion. Exp Parasitol. 1998;89:1–8. doi: 10.1006/expr.1998.4274. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Lanzer M. Changing ideas on chloroquine in Plasmodium falciparum. Curr Opin Infect Dis. 2000;13:653–658. doi: 10.1097/00001432-200012000-00013. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer W. Epidemiology of drug resistance in malaria. Acta Trop. 1994;56:143–156. doi: 10.1016/0001-706X(94)90060-4. [DOI] [PubMed] [Google Scholar]