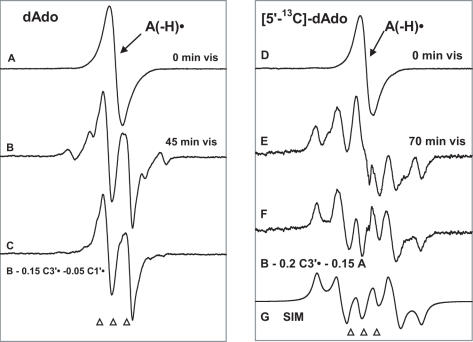
Figure 3.
(A) ESR spectrum of A(-H)• formed by oxidation of dAdo in 7 M LiCl glass/D2O with Cl2•−. (B) Spectrum after illumination with visible light for 45 min at 143 K, containing contributions from 80% C5′•, 15% C3′• and 5% C1′•. (C) Spectrum found after subtraction of C1′• and C3′• components (Figure 1B and C) from spectrum (B). This doublet is attributed to C5′•. (D) ESR spectrum from A(-H)• in [5′-13C]dAdo in 7 M LiCl glass/D2O. (E) Spectrum after illumination for 70 min with visible light at 143 K. This spectrum is made up of 80% C5′• and 20% C3′•; both radicals possess substantial 13C couplings (Table 2 and Scheme 3). (F) Spectrum obtained after subtraction of simulated C3′• spectrum (20%) (Figure 1E) and of A(-H)• spectrum (15%) (Figure 1A) from (D). This spectrum is assigned to C5′• (see text). (G) Simulation of spectrum in (F) using the parameters given in the text and in Table 2 and Scheme 3. All spectra were recorded at 77 K.