Abstract
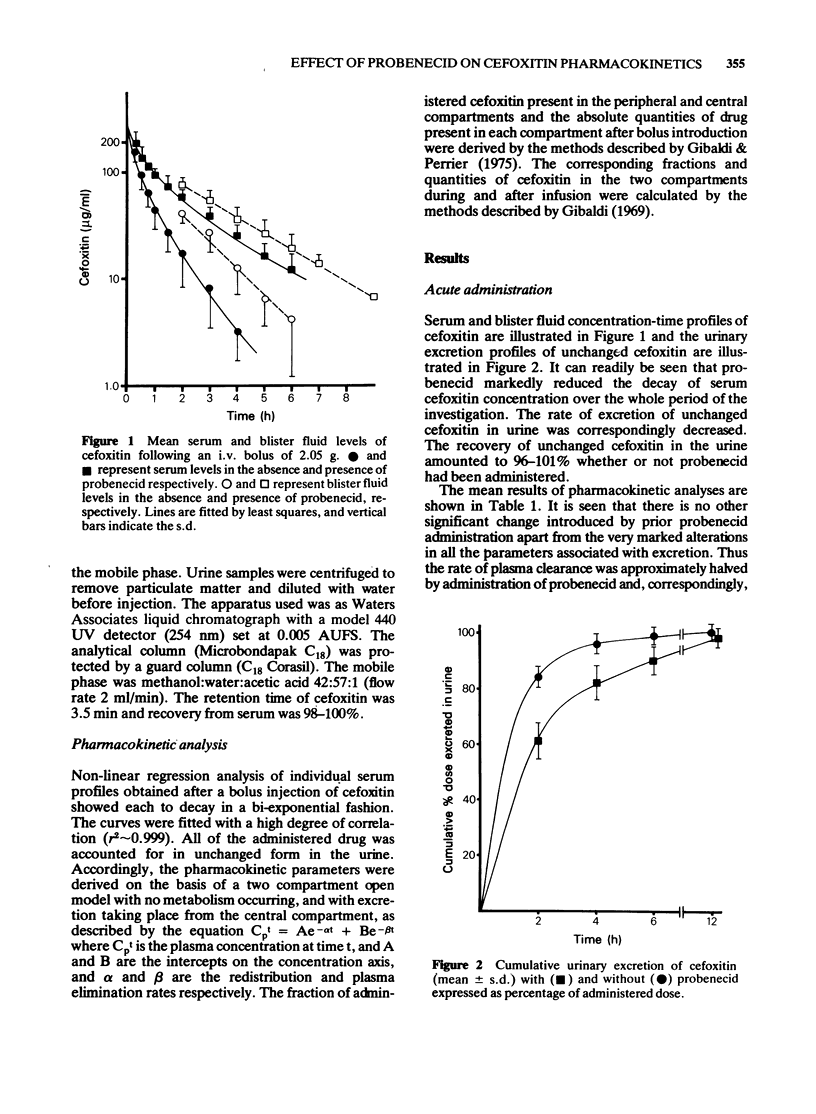
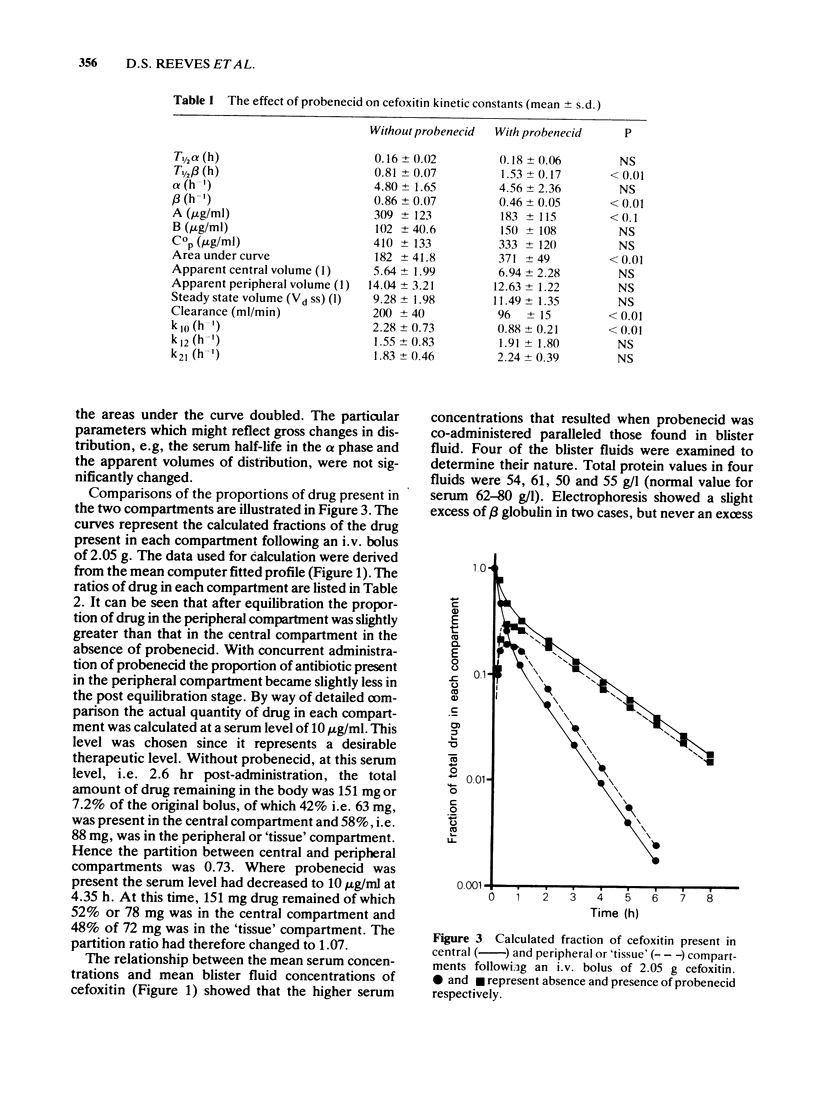
1 Cefoxitin was given by acute intravenous injection to six healthy volunteers, in a crossover study to investigate the effects of concurrent probenecid administration. 2 Serum antibiotic concentrations were determined by microbiological assay. Cefoxitin concentrations were simultaneously determined in the fluid of blisters produced by topical cantharides. All antibiotic was accounted for in the urine. 3 Cefoxitin was administered by intravenous infusion, subsequent to a loading dose, to produce steady state levels in the region of 10 microgram/ml, in one volunteer. The procedure was later repeated after prior administration of probenecid in the same subject. 4 Pharmacokinetic analyses indicated significant changes only in the parameters associated with renal excretion of drugs. Clearance was reduced by half. 5 The absolute and relative amounts of antibiotic in the central and peripheral compartments were calculated for both modes of administration. In the acute study probenecid produced a small change in distribution away from the peripheral or tissue compartment, towards the central compartment. 6 There was no elevation of initial serum concentrations and sustained levels of antibiotic could be accounted for principally by retarded excretion produced by probenecid, with little contribution by alteration in the disposition of antibiotic. 7 The sustained serum levels of cefoxitin that resulted from its decreased excretion were also reflected in blister fluid. It was concluded that the sustained cefoxitin levels produced by probenecid resulted in similar raised levels in the peripheral or "tissue' compartment, since the redistribution away from the peripheral compartment did not contribute materially to other changes in disposition of drug.
Full text
PDF



Selected References
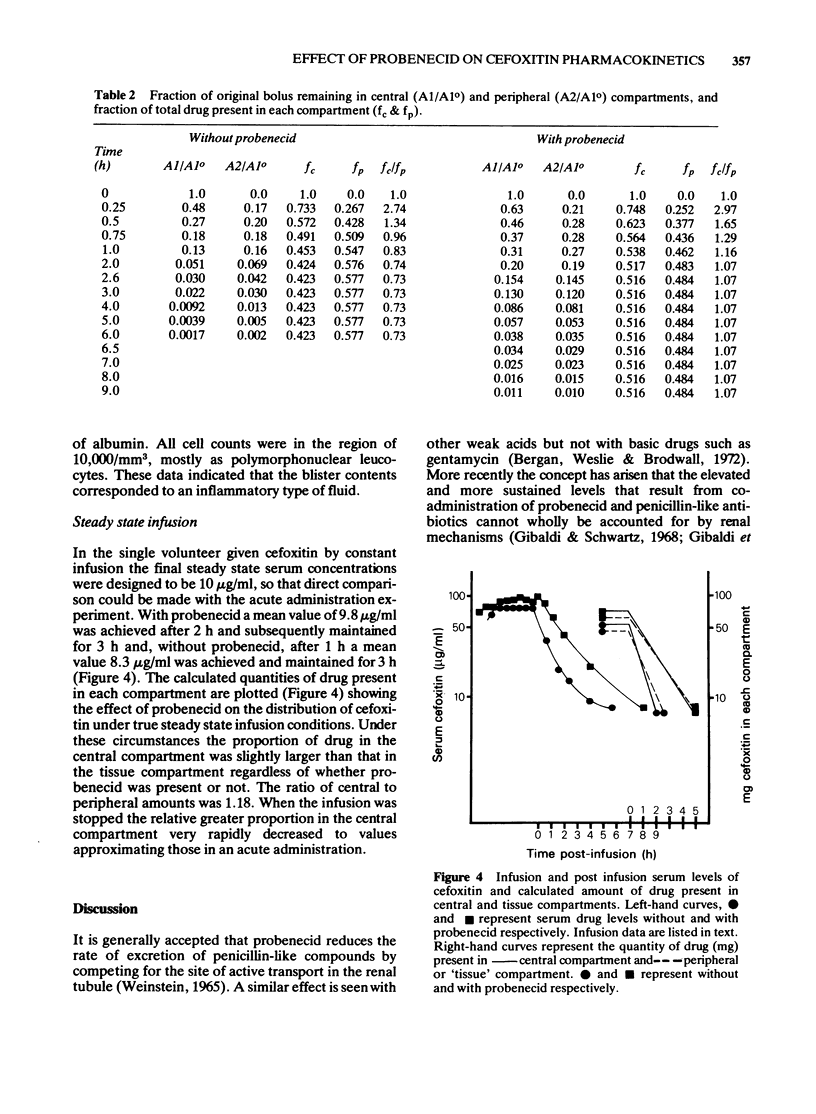
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum J., Stapley E. O., Miller A. K., Wallick H., Hendlin D., Woodruff H. B. Cefoxitin, a semi-synthetic cephamycin: a microbiological overview. J Antimicrob Chemother. 1978 Jul;4(B):15–32. doi: 10.1093/jac/4.suppl_b.15. [DOI] [PubMed] [Google Scholar]
- Geddes A. M., Wilcox R. M. Cefoxitin in abdominal sepsis. J Antimicrob Chemother. 1978 Jul;4(B):151–153. doi: 10.1093/jac/4.suppl_b.151. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Davidson D., Plaut M. E., Schwartz M. A. Modification of penicillin distribution and elimination by probenecid. Int Z Klin Pharmakol Ther Toxikol. 1970 May;3(2):182–189. [PubMed] [Google Scholar]
- Gibaldi M. Effect of mode of administration on drug distribution in a two-compartment open system. J Pharm Sci. 1969 Mar;58(3):327–331. doi: 10.1002/jps.2600580308. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Schwartz M. A. Apparent effect of probenecid on the distribution of penicillins in man. Clin Pharmacol Ther. 1968 May-Jun;9(3):345–349. doi: 10.1002/cpt196893345. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Raftery E. B., Goldberg A. D., Skeggs H., Till A. E., Martin C. M. Effects of rate of infusion and probenecid on serum levels, renal excretion, and tolerance of intravenous doses of cefoxitin in humans: comparison with cephalothin. Antimicrob Agents Chemother. 1974 Sep;6(3):338–346. doi: 10.1128/aac.6.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., Bint A. J., Holt H. A., Stocks P. J. Cefoxitin sodium: a clinical and pharmacological study. J Antimicrob Chemother. 1978 Jul;4(B):155–160. doi: 10.1093/jac/4.suppl_b.155. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J., Rowland M. Concept of a volume of distribution and possible errors in evaluation of this parameter. J Pharm Sci. 1968 Jan;57(1):128–133. doi: 10.1002/jps.2600570125. [DOI] [PubMed] [Google Scholar]
- Rodriguez N., Madsen P. O., Welling P. G. Influence of probenecid on serum levels and urinary excretion of cinoxacin. Antimicrob Agents Chemother. 1979 Mar;15(3):465–469. doi: 10.1128/aac.15.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C., Malerczyk V., Brahmstaedt E., Toeller W. Cefazolin, ein neues Breitspektrum-Antibiotikum. Dtsch Med Wochenschr. 1973 Dec 21;98(51):2448–2450. doi: 10.1055/s-0028-1107275. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Invest. 1974 Aug;54(2):316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling P. G., Dean S., Selen A., Kendall M. J., Wise R. Probenecid: an unexplained effect on cephalosporin pharmacology. Br J Clin Pharmacol. 1979 Nov;8(5):491–495. doi: 10.1111/j.1365-2125.1979.tb01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P., Leung T., Williams J. D. Antibacterial activity, pharmacokinetics and efficacy of cefoxitin in patients with abdominal sepsis and other infections. J Antimicrob Chemother. 1978 Jul;4(B):127–141. doi: 10.1093/jac/4.suppl_b.127. [DOI] [PubMed] [Google Scholar]


