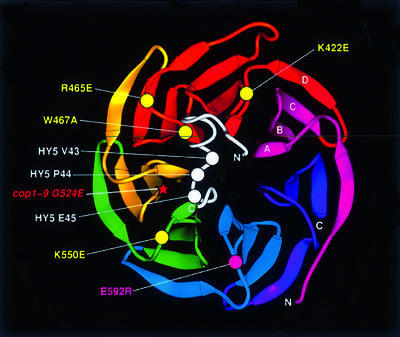
Fig. 9. A structural model for the interaction between the HY5 motif and the WD40 domain of COP1. The ribbons diagram (Carson, 1997) of the Gβ β-propeller was used to align the COP1 WD40 repeats. The carbon alpha backbone (Cα) positions of the substituted COP1 residues are marked by circles. Yellow circles indicate residues important for motif interaction whereas the magenta circle marks the mutation that had little or no effect. The star marks the position of the cop1-9 mutation. The COP1-interacting motif from HY5 (in white) is modeled based on the structure of homologous residues 213–239 in GCT (Jacob et al., 1997). The three key residues (V43, P44 and E45) are marked by white circles.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.