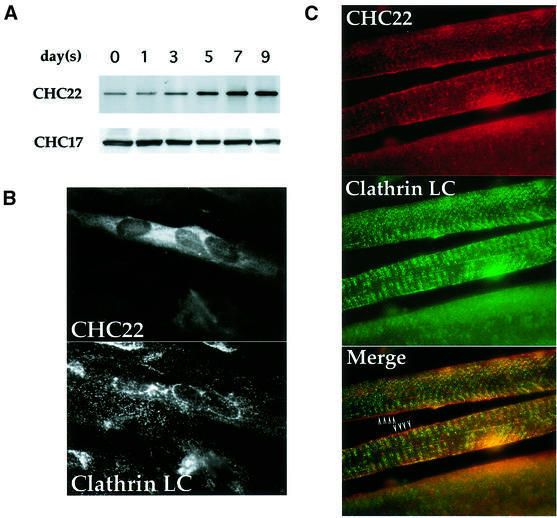
Fig. 1. Expression of CHC22 in human myoblasts and muscle tissue. (A) Induction of CHC22 during muscle differentiation. Human skeletal myoblasts SKMC were induced to differentiate in medium with low serum. After serum reduction, cells were harvested on the days indicated and CHC22 proteins were immunoprecipitated from cell lysates (adjusted to equal protein concentrations) using a CHC22-specific MAb. The immunoprecipitates were resolved by SDS–PAGE and CHC22 was detected with purified anti-CHC22 antiserum (CHC22) by immunoblotting. The expression levels of ubiquitous clathrin heavy chain CHC17 in the same lysates were detected by blotting with CHC17-specific MAb TD.1 (CHC17). (B) Immunofluorescence of differentiated human myoblasts. SKMC cells grown on collagen-coated coverslips were induced to differentiate as in (A) for 7 days in low serum medium. Cells were then analyzed by immunofluorescence for the distribution of CHC22, using a specific MAb followed by rhodamine (LRSC)-conjugated goat anti-mouse IgG, and for conventional CCVs (Clathrin LC) using anti-clathrin light chain antiserum α-cons followed by FITC-conjugated goat anti-rabbit IgG. Note that not all cells differentiated and that only multinucleated cells express detectable levels of CHC22. (C) Distribution of CHC22 and clathrin in human skeletal muscle. Tissue sections from normal human muscle were prepared for immunofluorescence and labeled for CHC22 (red) and clathrin light chains (Clathrin LC, green) as in (B). The three panels show the same sample viewed with different filters and the bottom panel shows the merged staining with both antibodies. Arrowheads indicate regions where lack of co-localization (separate red and green staining) is readily observed (see text), while a yellow signal indicates an overlap of staining patterns.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.