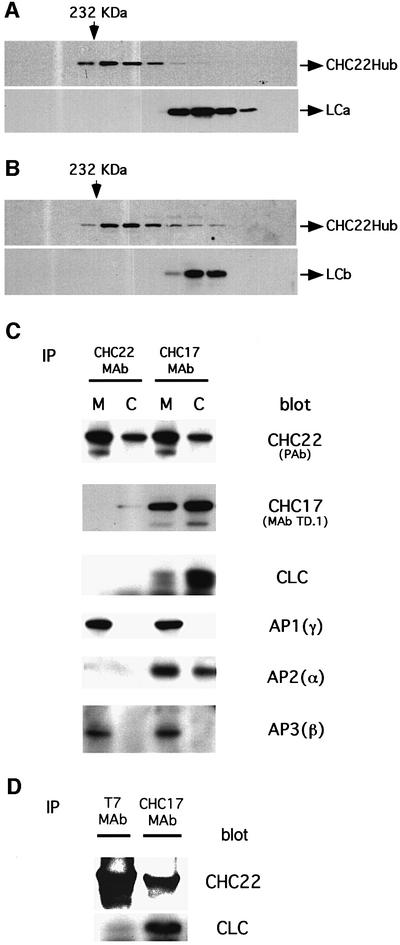
Fig. 2. Differential association of CHC22 with clathrin coat components. (A) Bacterial lysate containing recombinant CHC22Hub and co-expressed bovine neuronal LCa was separated by Superose 6 size exclusion chromatography. The column fractions were collected and resolved in sequence (left to right) by SDS–PAGE. The presence of CHC22Hub polypeptides or LCa was established by immunoblotting using rabbit serum against CHC22 (CHC22Hub) or MAb CON.1, which recognizes a determinant shared by both light chains (LCa and LCb). Arrows on the top indicate the fractions flanking the elution positions of molecular weight standard catalase (232 kDa). (B) Bacterial lysate containing recombinant CHC22Hub and co-expressed bovine neuronal LCb was separated by Superose 6 size exclusion chromatography and analyzed for the elution position of CHC22Hub polypeptides and LCb, as in (A). (C) Cytosolic and membrane-enriched fractions of HeLa 229 cells were prepared. CHC22 was then immunoprecipitated from each fraction using the MAb specific for CHC22. Conventional clathrin heavy chains (CHC17) were isolated with MAb X22 (Blank and Brodsky, 1986). The immunoprecipitates were then subjected to SDS–PAGE and probed with the following antibodies: anti-CHC22 polyclonal antiserum [CHC22 (PAb)], CHC17-specific monoclonal antibody TD.1, anti-clathrin light chain antiserum (CLC), anti-AP1 γ subunit monoclonal antibody 100/3 [AP1(γ)], anti-AP2 α subunit AC1M11 [AP2(α)] and anti-AP3 β3 antiserum [AP3(β)]. The CHC22 signals in CHC17 immunoprecipitates are due to cross-reactivity of X22 with CHC22, causing co-precipitation of CHC22 with CHC17 and possibly explaining the apparent association of AP3 with CHC17. (D) HeLa-tet/on cells were permanently transfected with T7-epitope-tagged full-length CHC22, under the tet operator (pJM601CHC22), and CHC22 expression was induced for 24 h with doxycycline. CHC22 full-length protein was then immunoprecipated using anti-T7 MAb and the sample was analyzed by SDS–PAGE and immunoblotting for CHC22 using a specific polyclonal antiserum, or for clathrin light chains (CLC) using the α-cons polyclonal antibody. Conventional clathrin (CHC17) was immunoprecipitated from the same sample using MAb X22 and similarly analyzed. Note that X22 immunoprecipitates some CHC22 along with CHC17 due to cross-reactivity (see C).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.