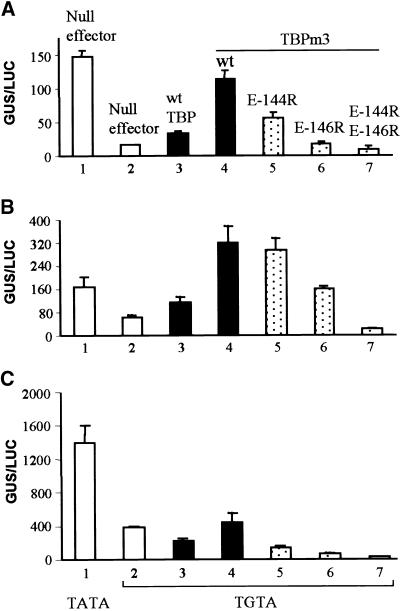
Figure 6.
Role of the C-Terminal Stirrup of TBP in Activated Transcription Evaluated by Using the Altered-Specificity System (TGTA/TBPm3).
(A) Transcription was activated by Gal4 DNA binding domain ftzQ. AtTBP2 was mutated by three amino acid substitutions within its concave DNA binding surface to generate TBPm3 (Strubin and Struhl, 1992). Transcriptional activity was monitored with the Gal4 (TGTA)–GUS reporter (Figure 3A, construct 6) in transient assays with maize cells. The activator was coexpressed with wild-type (wt), single, or double point mutations of TBPm3 as indicated. Quantities of DNA used in transformations remained constant. Bar 1 represents the control for normal activity as determined by using the wild-type TATA reporter and the endogenous TBP. The TGTA reporter (Figure 3A, construct 6) was used for experiments in bars 2 to 7. Activity, given as relative GUS/LUC units, is an average of triplicate assays. Standard errors are indicated.
(B) Transcription was activated by the Gal4 DNA binding domain–Gal4 activation domain. Experiments were conducted as described in (A).
(C) Transcription was activated by Gal4 DNA binding domain–VP16. Experiments were conducted as described in (A).