Abstract
The peroxisome proliferator-activated receptor-γ (PPAR-γ) is a prototypical metabolic nuclear receptor that acts as a lipid sensor, integrating the homeostatic control of energy, lipid, and glucose metabolism. This perspective will highlight three lines of evidence which place PPAR-γ as a key player in a feed-forward pathway favoring differentiation and energy storage by adipocytes.
PPAR-γ: a thrifty transcription factor co-ordinating adipogenesis and glucose homeostasis
The peroxisome proliferator-activated receptor-γ (PPAR-γ) is a prototypical metabolic nuclear receptor that acts as a lipid sensor, integrating the homeostatic control of energy, lipid, and glucose metabolism. PPAR-γ activity is governed by binding of small lipophilic ligands, mainly fatty acids, derived from nutrition or metabolic pathways that are often controlled by PPAR-γ. The actions of PPAR-γ are mediated by two protein isoforms, the widely expressed PPAR-γ1 and adipose tissue-restricted PPAR-γ2, produced by alternative splicing and differing only by 28 additional NH2-terminal residues in PPAR-γ2. Three main lines of evidence establish that PPAR-γ is the centerpiece of a feed-forward pathway that favors differentiation and energy storage by adipocytes and coordinates the “thrifty response”. [Auwerx, 1999; Chawla et al., 2001; Francis et al., 2003; Lee and Evans, 2002; Michalik and Wahli, 1999; Picard and Auwerx, 2002; Rosen and Spiegelman, 2001; Rosen et al., 2000; Sporn et al., 2001].
Molecular and cellular studies
The initial suggestion that PPAR-γ stimulated adipogenesis was based on the observation that expression of PPAR-γ in cells was by itself sufficient to induce adipocyte differentiation. Conversely, targeted mutagenesis of the PPAR-γ gene in ES cells and knocking down the endogenous PPAR-γ2 in cell lines confirmed the commanding role of PPAR-γ in adipocyte differentiation. Consistent with this, PPAR-γ increases genes that promote fatty acid storage, whereas it represses genes that induce lipolysis and fatty acid release from adipocytes.
Mouse and human genetic studies
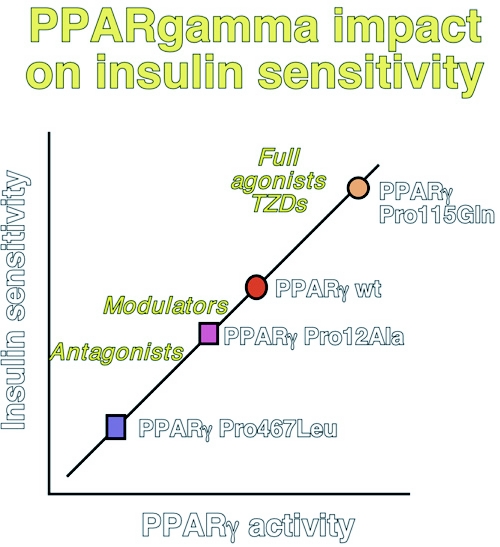
The lipodystrophy in the rare homozygous PPAR-γ -/- mice, together with the characterization of mice chimeric for PPAR-γ -/- ES cells, has unequivocally demonstrated the importance of PPAR-γ in adipose tissue development in vivo. Interestingly, PPAR-γ +/- mice are resistant to obesity and are more insulin sensitive. Further support for an important role of PPAR-γ in adipogenesis has come from human genetic studies, which have linked the PPARG locus on 3p25-p24 with obesity in Pima Indians. Furthermore, the partial loss-of-function Pro12Ala mutation in the PPAR-γ2-specific B exon lowers body mass, and improves insulin sensitivity and lipid profile. The association between the hypomorphic Ala substitution and insulin sensitivity disappears when the data are corrected for body mass, suggesting a primary effect on fat mass. In contrast to this hypomorphic allele, the rare Pro115Gln substitution constitutively activates PPAR-γ by blocking the Ser114 PPAR-γ MAP kinase phosphorylation site. In keeping with PPAR-γ′s adipogenic role, carriers of the Pro115Gln gain-of function mutation are obese and insulin resistant. The data on the Pro12Ala and Pro115Gln mutations were, however, somewhat in contrast with two dominant negative mutations, i.e. Pro467Leu and Val290Met, that have been associated with partial lipodystrophy, but also with severe insulin resistance, diabetes and hypertension (Figure 1).
Figure 1. Hypothesis summarizing the relationship between PPAR-γ activity, adipogenesis and glucose homeostasis.
This scheme is based on both genetic (Pro115Gln, Pro12Ala, Pro467 Leu) and pharmacological evidence (agonists, modulators or partial agonists, and antagonists). Only human mutations are shown for simplicity, but studies in mouse mutants support the human data. Higher PPAR-γ activity translates into insulin sensitization, but is associated with increased fat mass accretion. This underscores the importance of PPAR-γ modulation - or even antagonism - as the preferred strategy to combat the metabolic syndrome.
Pharmacological studies
PPAR-γ agonists, such as the thiazolidinediones, invariably increase white adipose tissue (WAT) mass, redistribute WAT from visceral to subcutaneous depots, and induce the appearance of small, newly differentiated adipocytes at the expense of large, mature adipocytes. Whereas enhanced PPAR-γ activity is associated with an increase in WAT, suboptimal PPAR-γ activation or PPAR-γ antagonism is neutral or even reverses weight gain. Partial PPAR-γ agonists or modulators, such as FMOC-L-leucine, NC-2100 or MCC-555, are less adipogenic but lower glucose more effectively then full PPAR-γ agonists in vivo. This effect is, at least for some of these partial agonists, linked to the recruitment of a distinct set of coactivators to PPAR-γ. Furthermore, inhibition of PPAR-γ /RXR activity by antagonists also translates into improved insulin sensitivity in vivo. These pharmacological studies are fully consistent with the effects of the partial loss of PPAR-γ function in both PPAR-γ +/- mice and carriers of the Pro12Ala PPAR-γ substitution (Figure 1).
The evidence reviewed above underscores the importance of PPAR-γ in adipogenesis, but fails to provide information on the role of PPAR-γ in adult physiology. WAT is required for proper glucose homeostasis, since lipodystrophy leads to severe insulin resistance. The observation that PPAR-γ agonists increase fat mass along with improving glucose control supports the notion that WAT mediates some of their effects on glucose homeostasis. A reduction in circulating free fatty acids, by repartitioning them towards fat rather than muscle, is an early consequence of PPAR-γ activation, and precedes the decrease in glucose and triglyceride levels. Besides fatty acids, WAT produces several adipokines, which affect insulin signaling in other tissues, and whose expression is also altered by PPAR-γ activation. Examples are TNF-α, leptin, and resistin, secreted proportionally with WAT mass, and adiponectin, whose levels are inversely related to the amount of WAT.
The role of PPAR-γ in the control of glucose homeostasis likely extends beyond its primary effects in WAT, and effects of thiazolidinediones have been reported in muscle, pancreatic β cells, and liver. These data are, however, potentially compromised by the specificity of the ligand. The importance of PPAR-γ in other tissues was, however, recently underscored by decreased hepatic trigylcerides levels and aggravated systemic insulin resistance in mice with a liver-specific knockout of PPAR-γ.
PPAR-γ from inflammation to atherosclerosis
In addition to these thrifty activities in WAT, other PPAR-γ activities, such as its role the differentiation of macrophages, crucial components of the innate immune system, might also be evolutionary beneficial. PPAR-γ induced in monocytes following exposure to oxidized LDLs (oxLDLs) activates the expression of the CD36 scavenger receptor, which increases oxLDL uptake and provides the cells with natural PPAR-γ agonists. Such a feed-forward cycle would predict that PPAR-γ is predominantly pro-atherogenic, were it not effectively counterbalanced by PPAR-γ -mediated reduction of inflammatory cytokine production by macrophages, which is clearly anti-atherogenic. Moreover, the expression of ABCA1, a transporter involved in apoA1-mediated cholesterol efflux from macrophages, is tightly regulated by the cellular content of cholesterol, through oxysterol-dependent activation of another nuclear receptor, the liver X receptor (LXR). PPAR-γ has been shown to activate ABCA1 expression indirectly via enhanced transcription of LXR. The efficacy of PPAR-γ agonists to reduce atherosclerosis is hence most likely a consequence of both anti-inflammatory and indirect LXR-mediated effects. The current epidemiological increase in atherosclerosis could be considered as a PPAR-γ -driven maladapted response in the macrophages, which occurs when the inherent beneficial effects (stimulation of the innate immune response and cholesterol efflux) are overwhelmed by the pro-atherogenic effects (increased OxLDL uptake). Such conditions are potentially met by the chronic overload of lipids characteristic of current affluent lifestyles in the Western Hemisphere.
PPAR-γ, caloric restriction, fat and aging
From an evolutionary perspective, a “thrifty response”, clearly favors survival. In apparent contradiction, caloric restriction, referring to a dietary regimen low in calories without undernutrition, is also known to extend lifespan in organisms, ranging from yeast to non-human primates. The beneficial effects of caloric restriction have also been associated with alterations in metabolism, more particular the insulin/insulin-like growth factor 1 (IGF-1) pathways and a decreased fat mass. These pathways converge on transcription factors of the forkhead family (Daf16 in the worm and FOXO1-3 in mammals), which are activated when insulin/IGF-1 signaling decreases, translating ultimately into the increased stress resistance, the hallmark of caloric restriction. The most plausible hypothesis explaining the anti-aging effects of caloric restriction is that a reduced flow of carbon through the glycolytic pathways will slow down the rate of conversion of NAD to NADH. The altered NAD/NADH ratio will then be recognized by neuro-endocrine sensors that reduce the production of growth hormone by the pituitary (and in turn also IGF-1) and insulin by the pancreas. The importance of insulin/IGF-1/FOXO signaling in longevity is supported by the extended lifespan of humans, mice, C. elegans, and Drosophila with mutations in this signaling cascade. It is at present unclear how PPAR-γ interfaces with this signaling pathway, but the fact that PPAR-γ coordinates adipogenesis and glucose homeostasis, leads us to speculate that it also may affect longevity.
Too much of a good thing …. harms
All this suggests that moderate (or partial) levels of PPAR-γ activation coordinate an evolutionary beneficial and adaptive response (Figure 2). Longevity is improved because efficient energy conservation and storage allows survival of episodes of food shortage, and an enhanced innate immune response helps to combat infections and uncontrolled cell proliferation. Our current affluent life style, which exposes us to excessive levels of natural PPAR-γ activators, brings this tightly regulated system out of balance. Being born with a silver spoon in our mouth now turns this once favorable response into a detrimental one, which contributes to the pathogenesis of life-style associated diseases, such as obesity, type 2 diabetes, and atherosclerosis. This also indicates that modulating (or inhibiting) PPAR-γ activity, rather than activating, might be the preferred therapeutic strategy to treat the above disorders.
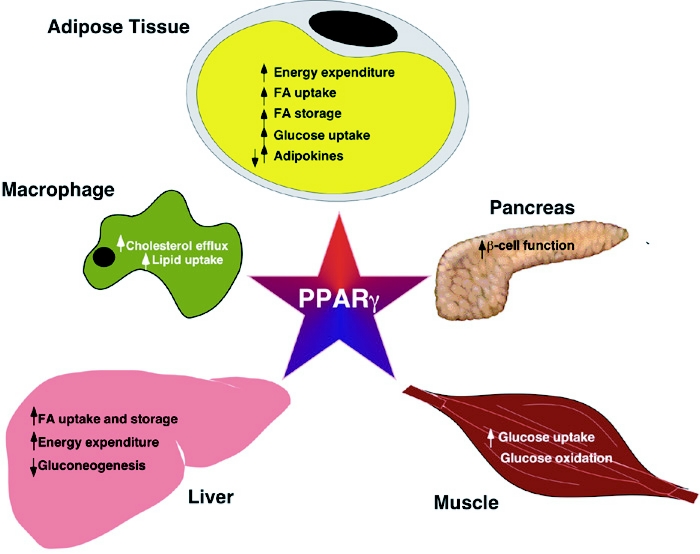
Figure 2. Physiological effects of PPAR-γ activation.
PPAR-γ induces adipocyte differentiation and genes involved in fat deposition. Plasma-derived fatty acids are directed to adipose tissue at the expense of skeletal muscle, which increases glucose uptake and utilization in the muscle. Direct effects of PPAR-γ activation have also been observed in liver, including decreased gluconeogenesis and increased fat uptake and storage. Additionally, PPAR-γ activation results in increased cholesterol efflux in macrophages via upregulation of ABCA1, but also increased uptake of proatherogenic oxidized LDL particles via upregulation of CD36.
Abbreviations
- OxLDL
oxidized low density lipoproteins
- PPAR-γ
Peroxisome proliferator activated receptor-γ
References
- Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Francis G. A., Fayard E., Picard F., Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Evans R. M. Peroxisome proliferator-activated receptor-γ in macrophage lipid homeostasis. Trends Endocrinol Metab. 2002;13:331–5. doi: 10.1016/s1043-2760(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Michalik L., Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Curr Opin Biotechnol. 1999;10:564–70. doi: 10.1016/s0958-1669(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Picard F., Auwerx J. PPAR(γ) and glucose homeostasis. Annu Rev Nutr. 2002;22:167–97. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Spiegelman B. M. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Walkey C. J., Puigserver P., Spiegelman B. M. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–307. [PubMed] [Google Scholar]
- Sporn M. B., Suh N., Mangelsdorf D. J. Prospects for prevention and treatment of cancer with selective PPARgamma modulators (SPARMs) Trends Mol Med. 2001;7:395–400. doi: 10.1016/s1471-4914(01)02100-1. [DOI] [PubMed] [Google Scholar]