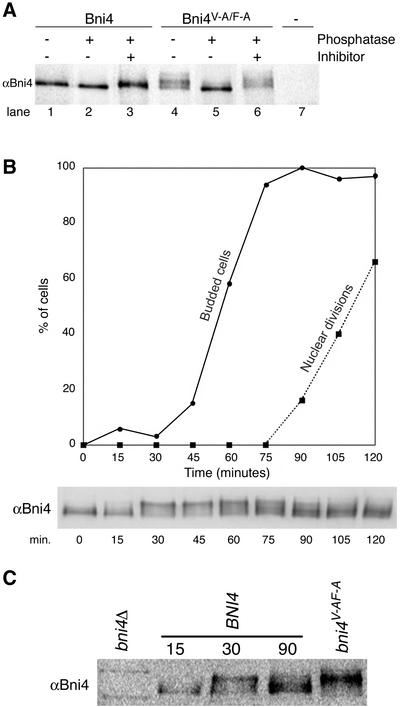
Figure 8.
Bni4 is covalently modified. (A) Bni4 is phosphorylated and Bni4V-A/F-A is hyperphosphorylated. Immunoprecipitation with anti-Bni4 antibody (α-Bni4) was performed on lysates from cells expressing either Bni4 (lanes 1–3), Bni4V-A/F-A (lanes 4–6), or cells disrupted for BNI4 (lane 7) (strains YLK74, YLK76, and YLK78, respectively). Precipitates were MOCK-treated (lanes 1 and 4), treated with calf intestinal phosphatase (lanes 2 and 5), or treated with the phosphatase and the vanadate inhibitor (lanes 3 and 6). Samples were electrophoresed, blotted to membrane, and probed with α-Bni4 antibody. (B) Phosphorylation of Bni4 is cell cycle-dependent. Cells (DLY222) were arrested in G1 by incubation in medium containing sucrose. The arrest was released by exposing the cells to galactose. Samples were collected every 15 min and examined for budding (solid line), nuclear division (dotted line), and Bni4 mobility on PAGE (immunoblot), as detailed in the text. (C) Electrophoretic mobility of Bni4 in the samples collected at 30 min is similar to that of Bni4V-A/F-A from an asynchronous culture. Immunoblot with anti-Bni4 was done following SDS-PAGE separation of 15, 30, and 90 min. samples along with extract from the strain YLK76.