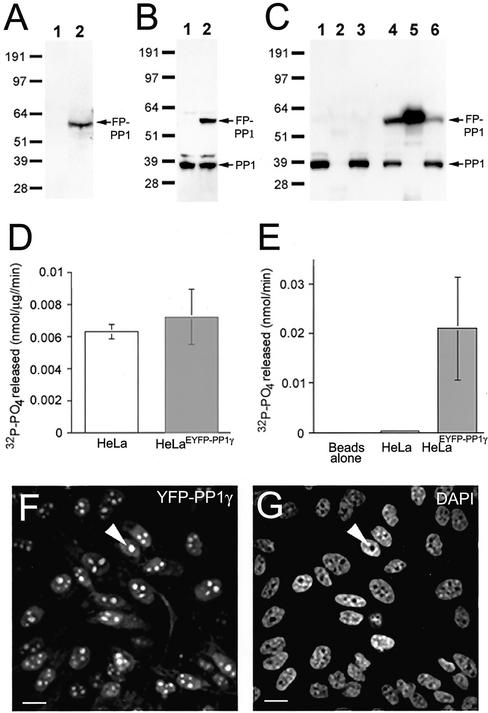
Figure 1.
HeLaEYFP-PP1γ cells express full-length, active fusion protein. (A) Lysates (30 μg total protein) from HeLa (1) and HeLaEYFP-PP1γ (2) cells probed on a Western blot with anti-GFP antibodies. (B) Similar lysates probed with anti-PP1 antibodies. (C) HeLa and HeLaEYFP-PP1γ cell lysates before (1 and 4) and after (3 and 6) incubation with anti-GFP antibodies coupled to protein G sepharose. Anti-PP1 antibodies detect endogenous PP1 in both lysates and the fusion protein in the HeLaEYFP-PP1γ lysate. The fusion protein was specifically immunodepleted by >50% from HeLaEYFP-PP1γ lysates (5), whereas no PP1-positive bands were pulled down from HeLa lysates (2). (D) Total in vitro phosphorylase, a phosphatase activity associated with HeLa and HeLaEYFP-PP1γ cell lysates. (E) Phosphatase activity associated with anti-GFP beads alone and with anti-GFP beads incubated with 50 μg total protein from HeLa or HeLaEYFP-PP1γ cell lysates. Data are means ± SD for n = 4. The EYFP signal in a field of HeLaEYFP-PP1γ cells is shown in F, with the DAPI-stained DNA pattern in G. A nucleolus is marked by the arrowhead. Scale bar, 10 μM.