Abstract
Objective:
To evaluate the effects of surgical weight loss on fatty liver disease in severely obese patients.
Summary Background Data:
Nonalcoholic fatty liver disease (NAFLD), a spectrum that extends to liver fibrosis and cirrhosis, is rising at an alarming rate. This increase is occurring in conjunction with the rise of severe obesity and is probably mediated in part by metabolic syndrome (MS). Surgical weight loss operations, probably by reversing MS, have been shown to result in improvement in liver histology.
Methods:
Patients who underwent laparoscopic surgical weight loss operations from March 1999 through August 2004, and who agreed to have an intraoperative liver biopsy followed by at least one postoperative liver biopsy, were included.
Results:
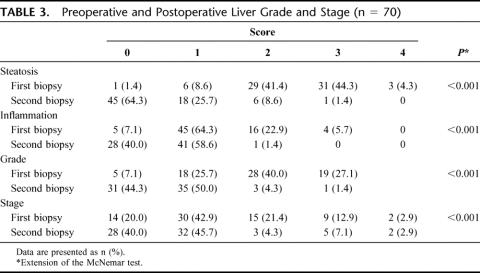
There were 70 patients who were eligible. All patients underwent laparoscopic operations, the majority being laparoscopic Roux-en-Y gastric bypass. The mean excess body weight loss at time of second biopsy was 59% ± 22% and the time interval between biopsies was 15 ± 9 months. There was a reduction in prevalence of metabolic syndrome, from 70% to 14% (P < 0.001), and a marked improvement in liver steatosis (from 88% to 8%), inflammation (from 23% to 2%), and fibrosis (from 31% to 13%; all P < 0.001). Inflammation and fibrosis resolved in 37% and 20% of patients, respectively, corresponding to improvement of 82% (P < 0.001) in grade and 39% (P < 0.001) in stage of liver disease.
Conclusion:
Surgical weight loss results in significant improvement of liver morphology in severely obese patients. These beneficial changes may be associated with a significant reduction in the prevalence of the metabolic syndrome.
The incidence of nonalcoholic fatty liver disease, one of the most common causes of end-stage liver failure, is rising rapidly in conjunction with the increase in severe obesity. Surgically-induced weight loss results in significant improvement in liver morphology, probably as a result of the amelioration of the metabolic syndrome.
Resolutely, the incidence of obesity continues its steady rise towards most-lethal disease status. If the current trend continues, 40% of the US population will be obese by the year 2025.1 Other indicators, such as the finding that 15% of children and adolescents are obese (body mass index [BMI] >95th percentile on the Centers for Disease Control and Prevention standard charts) and over 20% are “at risk” (BMI >85th percentile) are even more alarming harbingers of an impending health crisis.2 Obesity is associated with numerous comorbid factors (most of which are life-threatening) and these disease processes, not unexpectedly, are also on the rise. One of these complexes, nonalcoholic fatty liver disease (NAFLD), now occurs in a range of 30% to 100% of obese adults.3 Remarkably, it is prevalent in 53% of obese children.4
NAFLD has become the most common cause of liver disease and has reached epidemic proportions in developed countries. NAFLD is a spectrum that is initiated with steatosis; it can progress to nonalcoholic hepatosteatitis (NASH) and later fibrosis, cirrhosis, and potentially end-stage liver failure and/or hepatocellular carcinoma, in the absence of chronic alcohol use or other liver disease. Metabolic syndrome is present in 60% of females and 30% of males with NAFLD and this association rises with increasing BMI, from 18% in lean individuals to 67% of obese patients.5 Although the exact mechanism of liver injury is unknown, it is widely accepted that the metabolic syndrome is directly linked to fatty liver disease. The hallmark of metabolic syndrome is insulin resistance, as exemplified by Type 2 diabetes mellitus, hyperinsulinemia, hypertension, hyperlipidemia, and, in many patients, excessive visceral adiposity.
Intuitively, it follows that weight loss should be a therapeutic factor for NAFLD and, indeed, this been demonstrated previously by several investigators.6–9 To date, surgical solutions to morbid obesity have proven to be the most effective options for marked and sustained weight loss beyond 10 years.10 Although there have been reports of overall reduced prevalence and severity of liver disease after bariatric surgery, some of these studies were confounded by unexplained progression of disease in some postoperative patients.3,8
In this study, we set out to investigate our outcomes of laparoscopic weight loss procedures (gastric bypass, sleeve gastrectomy, and LapBand) in terms of postoperative changes in liver disease. We were particularly interested in determining the effect of weight loss on the components of metabolic syndrome and, by extension, on NAFLD as indicated by histologic confirmation in morbidly obese patients.
PATIENTS AND METHODS
The study was conducted in accordance with the ethical guidelines of the 1975 Helsinki Declaration and the protocol was approved by the ethics and research committees of our institution. From our prospectively designed electronic database, the University of Pittsburgh Bariatric Surgery Clinical Database (Access, Microsoft), we identified a subgroup of patients who underwent weight-loss surgery with a diagnosis of NAFLD, and who agreed to undergo serial liver biopsies for clinical assessment of their liver disease. Data sources included office charts, follow-up notes, hospital charts, and patient interviews. Parameters included patient demographics, BMI, comorbidity, weight loss, and change in comorbidity. We also analyzed the effect of weight loss on the status of diabetic and hypertensive patients, with special attention to the modification of relevant medications. Laboratory assessment of parameters of hyperlipidemia and liver function were also compared after weight loss. Inclusion criteria included patients with elevated liver function tests, gross features of fatty liver as depicted by ultrasonographic interrogation or intraoperative visual assessment of the liver, or histologic evaluation of liver biopsy specimens obtained at the time of operation. Patients were excluded if they had history of alcoholism, consumed >20 g alcohol per day, had evidence of autoimmune hepatitis, chronic hepatitis B or C virus, HIV, genetic hemochromatosis, alpha 1 antitrypsin deficiency, Wilson disease, or were taking known hepatotoxic drugs. Other exclusionary criteria included patients in whom no repeat liver biopsy was performed, or if the time interval between initial and repeat liver biopsy was less than 3 months.
All patients were evaluated extensively, including history and physical examination, nutritional and psychiatric evaluation, and specialty consultations when indicated. All patients were screened for diabetes using American Diabetes Association criteria; including fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L). Impaired fasting glucose was defined as FPG between 100 and 125 mg/dL.11 Comorbidities associated with diabetes and obesity were recorded. For the purposes of this study, the Adult Treatment Panel (ATP) III criteria were used to diagnose metabolic syndrome.12 The six conditions associated with metabolic syndrome are abdominal obesity, atherogenic dyslipidemia, hypertension, insulin resistance, a prothrombotic state, and a proinflammatory state; diagnosis is based on the presence of at least three of these conditions.
Preoperative studies included complete blood count, urinalysis, serum chemistries, nutritional indices, pregnancy test (in women younger than age 50), electrocardiogram, chest roentgenogram, and abdominal sonogram. Liver function tests were analyzed on a Dade-Behring Dimension RXL Chemistry analyzer (Deerfield, IL). Patient specimens were sampled by the analyzer into individual cuvettes where test specific reagent was added. After a specific incubation time, the analyte concentrations were read photometrically by the analyzer. Measured parameters included alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin. Also measured were biochemical components of the lipid panel including total cholesterol, serum triglycerides, high density lipoproteins (HDL), and low density lipoproteins (LDL).
Patient preparation for operation consisted of a detailed explanation in written and oral form of the weight loss operation, its benefits, alternatives, and risks including short- and long-term complications, side effects, nutritional sequelae, and the possibility of conversion to the open procedure. Written informed consent was obtained from all patients. Preoperative bowel cleansing and perioperative antibiotics were administered. Prophylaxis against venous thrombosis and pulmonary embolus consisted of perioperative pneumatic compression devices and low-dose subcutaneous heparin (5000 units, every 8 hours) or low molecular weight heparin 30–40 mg every 12 hours. All patients received postoperative marginal ulcer prophylaxis with antisecretory medications, in the form of H2 blockers for 3 months. Patients in possession of normal (acalculous) gall bladders also received ursodeoxycholic acid (UDCA; Actigall 300 mg bid) as a stabilizer of bile composition for 6 months after operation.
Surgical weight-loss operations were carried out by the laparoscopic approach in all patients. The operations included the Roux-en-Y gastric bypass (LRYGB), the laparoscopic adjustable gastric band (LAGB), and the sleeve gastrectomy (LSG). The operative technique for RYGB has been discussed elsewhere.13 Briefly, the gastric bypass is performed with the purpose of creating a 15-mL capacity gastric reservoir and either a 75-cm (short limb) or 150-cm (long limb) Roux segment of small intestine. The positioning of the Roux limb was modified over time, being retrocolic in the initial 850 patients and antecolic/antegastric in all subsequent patients. In the case of the LAGB, the pars flaccida technique was routinely used for placement of the band. Initial band adjustments were carried out, if needed, on the first postoperative visit, usually at 4–6 week.14 For LSG operations, these were performed by longitudinal resection of the stomach parallel to a F48 bogie advanced along the lesser curvature. The type of operation performed was based on patient preference and on surgeon decision in the course of surgery. Generally, patients considered to be at high operative risk were selected for sleeve gastrectomy, to return at a later date for the definitive laparoscopic gastric bypass after amelioration of their risks.
Liver biopsies were obtained intraoperatively and at follow up percutaneously with the use of the TruCut biopsy device (Bard Maxcore, Covington, GA). Intraoperatively, the TruCut device was advanced through the anterior abdominal wall in the region of the epigastrium, under laparoscopic vision, into the substance of the left lobe of the liver. Deployment of the device resulted in the acquisition of at least a 10-mm core of hepatic tissue. Repeat biopsies were immediately taken if specimens were unsatisfactory. At follow-up, liver biopsies were obtained either in the course of second operations such as laparoscopic exploration or laparoscopic cholecystectomy, or percutaneously, ultrasound-guided, in the gastrointestinal department by one of the authors (M.R.). A 15-gauge biopsy needle (Microvasive), which allows for at least 1-cm long core, was used for the ultrasound guided-technique.
The liver biopsies were processed routinely in the clinical histology laboratory. Formalin-fixed, paraffin-embedded histologic sections (4 ìm) were stained with hematoxylin and eosin (H&E), Masson Trichrome, and Prussian Blue (iron) stains for microscopic evaluation. The biopsies were evaluated by two experienced hepatopathologists who were blinded to patient characteristics and biopsy sequence. Three features of NAFLD/NASH were graded histologically according to the modified Brunt classification:15 steatosis, inflammation, and fibrosis. Steatosis was graded on a scale from 0 to 4 according to the amount of fat that was present throughout the lobules: 0, none (<1%); 1, 1–25%; 2, 26–50%, 3, 51–75% and 4, >75%. Inflammation was graded on a scale of 0–3: 0, none; 1, mild (scattered lymphocytes or small clusters within portal tracts and lobules); 2, moderate (same as grade 1 but with increased portal and lobular inflammation with lobular macrophages and/or neutrophils); and 3, severe (same as grade 2 but with more intense inflammation, including several collections of inflammatory cells in the lobules, concentrated around zone 3). Fibrosis was staged on a scale of 0–4: 0, none; 1, centrilobular pericellular fibrosis; 2, periportal and pericellular fibrosis; 3, bridging fibrosis; and 4, cirrhosis. Figure 1 illustrates some different grades of steatosis, inflammation, and fibrosis. In addition, the amount of iron that was present was graded on a scale of 0–4: 0, none; 1, accumulation of iron (hemosiderin) within periportal hepatocytes; 2, accumulation in zone 1 and some zone 2 hepatocytes; 3, accumulation in zones 1, 2 and some zone 3 hepatocytes; and 4, diffuse/massive accumulation in most/all hepatocytes.16
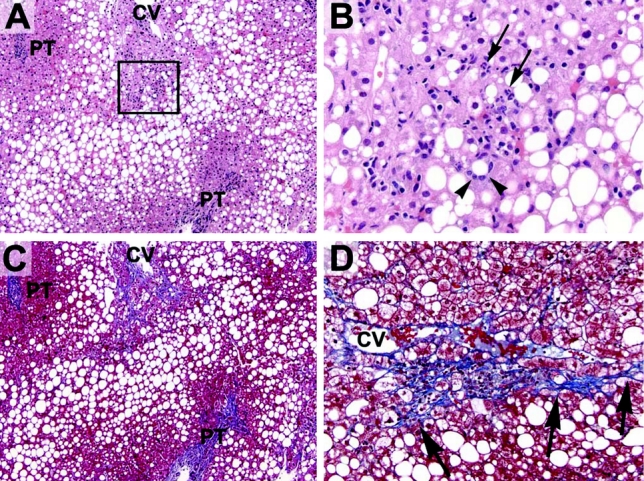
FIGURE 1. Representative histologic sections of a liver biopsy before bariatric surgery. A, Most preoperative livers contained abundant steatosis. In this example (H&E stain), macrovesicular steatosis is present throughout most of the lobules with relative sparing of the periportal hepatocytes (PT, portal tract; CV, central vein). B, Many preoperative livers also had evidence of steatohepatitis. The boxed area seen in (A) is shown here. Several hepatocytes are surrounded by inflammatory cells (one such hepatocyte is indicated by the arrowheads). The inflammatory infiltrate is composed of neutrophils (arrows), lymphocytes and macrophages (arrowheads). C, Many preoperative livers also had increased fibrosis, as highlighted here with a trichrome stain (same section as shown in A). Pericellular fibrosis is noted around the central vein (blue strands) without significant periportal fibrosis, indicating stage 1 fibrosis (usually, the central veins are free of any fibrosis). Hepatocytes are dark red with this stain. D, Higher power view of another area showing centrilobular pericellular fibrosis (emphasized by the arrows).
Patient data were collected prospectively, verified and then entered into the above-mentioned electronic database. Patient follow-up was scheduled for every 3 months with laboratory evaluation every 6 months until weight loss stabilization occurred, then at least once per year. Follow-up weights were obtained from the University of Pittsburgh Bariatric Surgery Clinic scale with a capacity of 1,000 lbs. Weight loss was expressed in terms of percent of excess weight loss (EWL). Ideal body weight was determined according to the Metropolitan Life Insurance Company 1983 height/weight tables; for a given height, the middle weight for a medium-frame person was chosen as the ideal body weight.
Statistical Analysis
Data are presented as means ± SD for normally distributed continuous variables and as percentages for categorical variables, or as median (interquartile range; 25th percentile to 75th percentile) for continuous variables lacking a normal distribution. For within-group comparisons (first vs. second liver biopsy), a paired Student t-test was used for parametric data and Wilcoxon sign rank test for nonparametric data. An extension of the McNemar test (marginal homogeneity test) was used to evaluate pre- (first biopsy) and postoperative (second biopsy) differences in NASH scoring. Comparisons between groups were performed using the unpaired t test and ANOVA for normally distributed variables, the Mann-Whitney and Kruskal-Wallis test for nonnormal variables, and the χ2 test and Fisher exact test, when appropriate, for categorical variables. Participants were divided into two groups; those with no change (second biopsy score –first biopsy score = 0) and with improvement (second biopsy score –first biopsy score <0) in NASH grade or score. Stepwise logistic regression analysis was used to assess independent predictors of improvement in NASH grade or stage. All statistical tests were two-tailed and P values of <0.05 were considered to be statistically significant. All analyses were performed using the Statistical Packages for Social Sciences (SPSS, version 11.5, SPSS, Chicago, IL).
RESULTS
Patient Characteristics
From March 1999 through August 2004, 3,312 patients underwent surgical weight loss operations at the Section of Minimally Invasive Surgery of University of Pittsburgh Medical Center. Of these, 72 patients met diagnostic criteria for NAFLD and agreed to a second liver biopsy. Two patients were excluded because of coexistent liver pathology (one patient with hepatitis C virus, one patient with Epstein-Barr hepatitis). There were 48 women and the mean age was 49 ± 9 years. The mean initial weight at time of operation was 339 ± 72 lb (154 ± 33 kg), corresponding to a BMI of 56 ± 11.
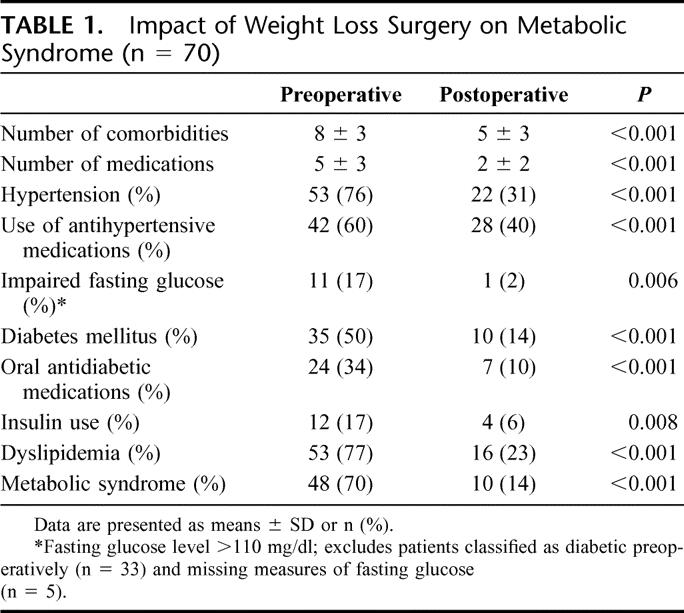
Table 1 shows that about two-thirds of the patients carried the diagnosis of metabolic syndrome and hypertension, whereas at least half the patients had type 2 diabetes mellitus. The number of comorbidities ranged from 3 to 15, with a mean of 7.6 comorbid factors per patient. Hypertensive patients had their blood pressure adequately controlled at the time of operation, taking one to four antihypertensive medications preoperatively, with a mean of 1.5 agents per day per patient. Diabetic patients were on zero to three oral antidiabetic medications, with a mean of 0.51 agents per day per patient. Twelve (17%) patients required insulin for glucose control preoperatively. Forty-two patients (60%) received postoperative UDCA as a prophylactic measure for gallstone formation. Liver function tests (LFTs) were not elevated in the majority of patients.
TABLE 1. Impact of Weight Loss Surgery on Metabolic Syndrome (n = 70)
Distribution of Operations
All patients underwent attempted laparoscopic surgical weight loss operations. LRYGB was performed in 41 patients, LSG in 23 patients, and six patients had laparoscopic placement of a LapBand. Patients who were deemed a high operative risk at the time of surgery were staged with an initial sleeve gastrectomy, followed by gastric bypass after an appropriate time interval, as previously discussed.17,18 Liver biopsies were taken intraoperatively at the time of all operations, including postoperative explorations and/or cholecystectomy. Biopsies were also taken percutaneously at follow-up in the Department of Gastrointestinal Medicine by one of the authors (M.R.).
Operative Outcomes
There were no deaths and the overall complication rate was 7%, including one patient who sustained a pulmonary embolus which required embolectomy, one patient with acute renal failure that resolved with intravenous hydration, one patient with a splenic abscess treated with percutaneous drainage, one acalculous cholecystitis treated with intravenous antibiotics, and one patient with a urinary tract infection who was treated with antibiotics.
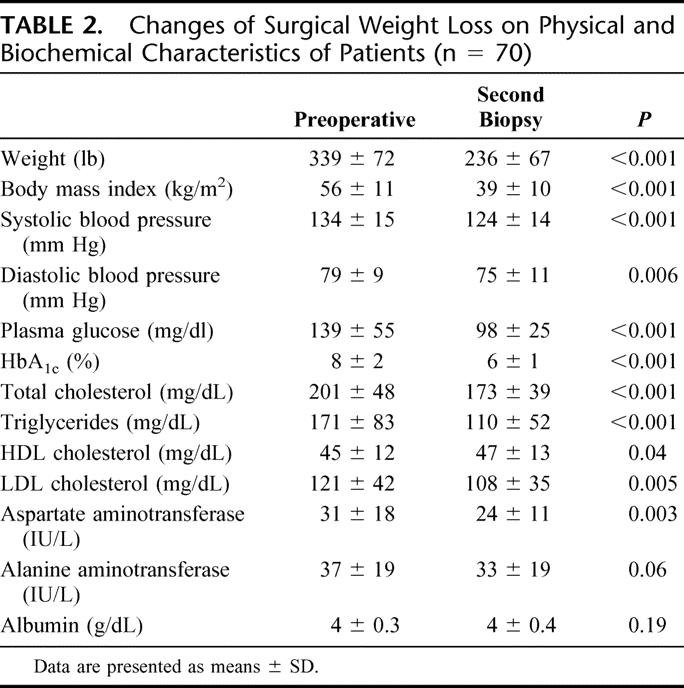
The time interval between liver biopsies was a mean of 15 ± 9 months. Table 2 shows that the mean weight of patients at the time of the second liver biopsy was 236 ± 66 lb, representing an excess body weight loss of 59% ± 22%. This weight loss had a profound beneficial effect in several areas. There were major improvements in the biochemical markers of metabolic syndrome, fasting blood glucose, and HbA1c. Following weight loss, fasting high density lipoprotein cholesterol was elevated while cholesterol and triglyceride concentrations were lowered. Liver function tests taken at the time of second biopsy as shown in Table 2 indicate either continued normal levels or improvement of these parameters. As for the possible beneficial role that UDCA administration may have played, a comparative analysis between this subgroup of 42 patients and the 28 patients who did not receive UDCA showed no difference in outcomes in terms of grade (P = 0.56) or stage (P = 0.33) of liver disease.
TABLE 2. Changes of Surgical Weight Loss on Physical and Biochemical Characteristics of Patients (n = 70)
Marked improvement, or resolution, was achieved with weight loss in most comorbid conditions. There was a significant diminution in the number of comorbidities. These included sleep apnea that was improved (37%) or resolved (37%), gastroesophageal reflux improved (20%) or resolved (52%), and joint pain/degenerative joint disease improved (37%) or resolved (40%), to name a few. Those diseases that persisted did improve, as indicated by a 50% reduction in the need for medications.
Histologic Assessment
All specimens were scored for NASH grade and stage as per guidelines mentioned above. Scores for liver steatosis, inflammation, and fibrosis were all significantly improved (Table 3). Of particular interest was the resolution of steatosis and inflammation in 37% of patients and resolution of fibrosis in 20% of patients, as indicated by the increased number of second liver biopsies that fell in the grade and stage 0 categories. None of the second biopsies revealed progression of grade or stage of liver disease. The two patients with cirrhosis (stage 4) had no improvement in fibrosis, but had improvement in steatosis and inflammation. Figures 2 and 3 are representative photomicrographs of the improvement in liver histology that can be demonstrated after weight loss.
TABLE 3. Preoperative and Postoperative Liver Grade and Stage (n = 70)
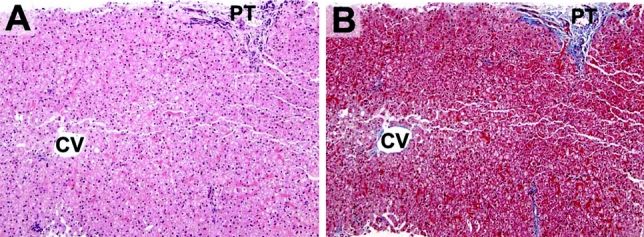
FIGURE 2. A liver biopsy from the same patient shown in Figure 1, now 13 months postbariatric surgery. A, There is no evidence of steatosis (PT, portal tract; CV, central vein) (H&E stain). B, There is no evidence of centrilobular fibrosis (Trichrome stain).
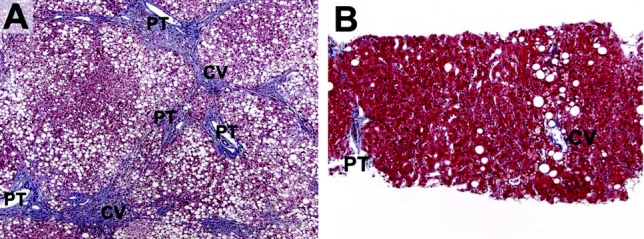
FIGURE 3. Another patient's liver biopsies pre- and postbariatric surgery. A, Preoperatively, the liver demonstrates diffuse (severe) steatosis and portal-to-central bridging fibrosis (wedge biopsy, Trichrome stain). B, Postoperatively (8.5 months postoperative), there is mild residual centrilobular steatosis and no evidence of significant fibrosis (trichrome stain).
Bivariate analysis revealed that preoperative AST levels (rs = 0.32, P = 0.008; for this and following analyses AST was log-transformed) and %EWL (rs = 0.27, P = 0.03) were associated with improvement in NASH grade score. Lower preoperative BMI (rs = 0.24, P = 0.01) was associated with improvement in NASH stage score. Logistic regression modeling of the total population resulted in the following variables being significantly independently associated with improvement in NASH grade (defined as a decrease in grade score): female sex (β = 2.67, se = 1.19, P = 0.02) and preoperative AST levels (β = 4.75, se = 2.37, P = 0.04). Statistically significant predictors of improvement in NASH stage were younger age (β = −0.10, se = 0.05, P = 0.05) and female sex (β = 1.51, se = 0.78, P = 0.05). Metabolic syndrome status preoperatively was not associated with improvement in NASH grade or stage. Variables in both models included age, sex, preoperative BMI, %EWL, and metabolic syndrome status preoperatively, as well as measures in Table 2 that were univariately associated with improvement in NASH grade and stage.
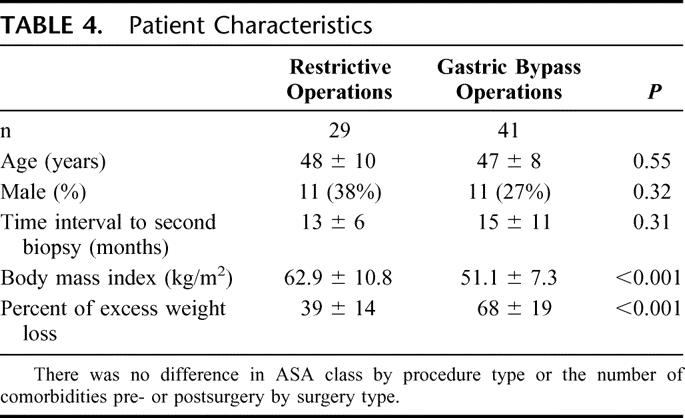
As expected, restrictive procedures (sleeve gastrectomies and LapBands) had a significantly less dramatic impact on weight loss than gastric bypass operations (Table 4). This correlated with a beneficial, albeit reduced, effect on liver morphology. Although there was universal weight loss and improvement in liver histology across all grades and stages, those patients who underwent gastric bypass operations had significantly better results. Improvement in grade in the restrictive group (66% showed improvement in grade) was significantly less than in the gastric bypass group (93%; P = 0.004). After adjusting for percent excess weight loss, type of procedure (gastric bypass versus restrictive) was no longer a statistically significant predictor of improvement in stage (P = 0.09, logistic regression analysis). There was no statistically significant difference in improvement in stage between the restrictive group (28%) and the gastric bypass group (46%; P = 0.11), although there was a trend for greater improvement in the gastric bypass group.
TABLE 4. Patient Characteristics
DISCUSSION
In this study of 70 patients who underwent repeat liver biopsy after dramatic weight loss, we demonstrate significant and widespread improvement, or resolution, of NAFLD and NASH. More than one-third of the patients had postoperative liver biopsies that showed resolution of steatosis and inflammation, and 20% of the patients had at least some reversal of fibrosis. No patient experienced a progression of abnormal liver morphology or a deterioration of hepatic function, as indicated by persistently normal liver enzymes. Our results highlight the important role that obesity and, by extension, the metabolic syndrome plays in the NAFLD disease process. Conversely, our data also show the profound beneficial effect that weight loss, possibly by the associated amelioration of comorbidities, has on the reversal of steatosis and fibrosis. Additionally, we have been able to achieve these outcomes safely, with no mortality and minimal morbidity.
The intimate relationship that obesity has with NAFLD is thought to be based on the presence of the metabolic syndrome, the hallmark of which is insulin resistance (IR). IR is, in fact, the common link between all etiologies of NAFLD.19 In average-risk individuals, IR, oxidative stress, and cytokine toxicity are the common factors that are involved in the pathogenesis of NAFLD and NASH. Severely obese patients have higher levels of IR, diabetes, oxidative stress, and circulating cytokines. Additionally, microsomal oxidative stress agents such as cytochrome P450 2E1 and P450 4A11, systemic continuous or intermittent hypoxemia (through sleep apnea), and nutritional toxicities can all contribute to the pathogenesis of NAFLD.20,21 Severely obese patients also consume large amounts of dietary fat resulting in higher levels of oxidative stress and lipid peroxidation, and higher levels of carbohydrates, which, in turn, can exacerbate insulin resistance.22 The resulting oxidative stress can lead to mitochondrial and DNA damage and initiation of the chronic inflammatory cascade of hepatocellular injury, activation of hepatic stellate cells characterized by their expression of α smooth muscle actin resulting in progressive fibrosis, and finally, cirrhosis.5
Histologically, the livers of patients with NAFLD can exhibit a spectrum of findings, ranging from isolated steatosis to steatosis plus inflammation, liver cell injury, and fibrosis. In the setting of steatosis, once liver cells become injured and liver enzymes begin to rise, the term steatohepatitis is applied. Liver biopsy interpretation is currently considered the “gold standard” for the diagnosis of steatohepatitis and the histologic features of steatohepatitis include a mixture of macrovesicular and microvesicular steatosis, lobular inflammation composed of lymphocytes, neutrophils and macrophages, hepatocyte injury often characterized by ballooning degeneration and increased, initially centrilobular pericellular fibrosis. As steatohepatitis persists and progresses, the amount (stage) of fibrosis also progresses from centrilobular pericellular fibrosis to periportal fibrosis and finally to cirrhosis. To promote uniform grading and staging of steatohepatitis by pathologists, a histologic grading and staging system has been developed and incorporates the findings described above.15
The beneficial effects of weight loss are believed to be mediated primarily via improved insulin sensitivity. In a recent article, Kral and colleagues postulated that sustained postoperative decrease in circulating glucose and insulin levels followed by the reduction in adipose mass and, concomitantly, reduced levels of leptin reduce fatty infiltration and inflammation, thereby restoring insulin sensitivity. The end result is the elimination of the fibrogenic tendencies of leptin, leading to reversal of fibrosis and cirrhosis.9
Available nonsurgical weight loss treatments include low-calorie and very low-calorie diets, exercise, behavioral modification, and pharmacotherapy. Although these modalities achieve modest and usually transient weight loss, there may be some benefit from applying pharmacotherapeutic strategies to patients with NAFLD. In a small pilot study of patients receiving Orlistat for 6 months, Harrison et al demonstrated improved steatosis and fibrosis in patients who lost at least 10% of body weight.23 Ursodeoxycholic acid (UDCA) may have therapeutic effects in patients with NASH, based on its presumed cytoprotective properties. Laurin et al reported the significant improvement of liver enzymes and liver morphology in patients with NASH after 12 months of UDCA administration.24 However, in a randomized, placebo-controlled study, UDCA treatment of 2 years failed to show a statistical significant difference between the UDCA- and the placebo-treated groups.25 When analyzing a subcohort of the study patients with prior cholecystectomy who did not receive UDCA, we were unable to expose any significant differences with the group of patients who received UDCA.
Surgical therapy, on the other hand, is the most effective solution for severe obesity in terms of extent and durability. The surgical armamentarium includes restrictive, hybrid restrictive/malabsorptive, and primarily malabsorptive operations. The most common operation performed for weight loss is the Roux-en-Y gastric bypass, considered by many to be the gold standard. This operation, which has the combined properties of restriction and malabsorption, has a proven track record of significant long-term weight loss with acceptable rates of mortality and morbidity.10 Other authors have also reported profound weight loss that was associated with improvement in NAFLD following gastric bypass.3,6 Purely restrictive operations such as the placement of an adjustable gastric band, which have largely supplanted vertical gastroplasty, have been used in patients with NAFLD, also with good outcomes.8 Although both strategies are effective in improvement of comorbidity, restrictive operations (when compared with malabsorptive procedures) result in less weight loss at a slower rate. Malabsorptive operations cause a larger weight loss at a rapid rate, but they are not without nutritional and metabolic consequences. Due diligence must be practiced in follow-up of these patients to prevent sequelae of nutritional deficiencies. Jejunoileal bypass, a widely popular operation for weight loss in the mid-1950s to mid-1970s, is now relegated to the history books due to the significant metabolic complications and liver injury that often culminated in cirrhosis.26
Although the exact mechanism is unclear, hepatocellular injury due to rapid weight loss is thought to be the result of metabolic stress. Rapid weight loss may actually aggravate liver function and liver histology as a result of increased free fatty acid level derived from extensive fat mobilization. One possible explanation may be that such vulnerable livers develop NASH when exposed to an additional factor, such as toxins from bacterial overgrowth or nutritional challenge that represent a “second hit.” These detrimental changes, however, appear to be reversible, as proven in patients who underwent dismantling of their jejunoileal bypasses. In a series of patients undergoing gastric bypass, Luyckx et al also confirmed this reversibility of liver histology by demonstrating that although steatosis improved, inflammation actually worsened (albeit transiently) improving over time in accordance with resolution of other metabolic abnormalities.3 Dixon et al demonstrated major improvement in liver disease in patients who achieved significant weight loss with the laparoscopic placement of the adjustable gastric band (LAGB).8 The most common primarily malabsorptive operation currently performed is the biliopancreatic diversion (BPD). In a recent article, the authors reported remarkable reversal of fibrosis and macroscopic liver appearance in cirrhotic patients after BPD. That series did, however, also reveal increased fibrosis in nearly 40% of patients, a development found by the authors to be associated with decreased serum albumin and poorly managed diarrhea, two known potential manifestations of BPD.9 That study also included three patients who developed de novo cirrhosis.
Many surgeons are reluctant to offer weight loss surgery to patients known to have liver cirrhosis, or indeed, abort procedures once an intraoperative diagnosis is made of advanced liver disease. We have previously reported on the safety and efficacy of minimally invasive weight loss surgery in cirrhotic patients.27 The present study not only confirms the beneficial results attainable in patients with advanced NAFLD but also presents histologic proof of reversal of liver disease. In a recent study, Fassio et al reported that, without treatment, liver fibrosis progresses in approximately one-third of patients with NASH and the presence of obesity is the only factor associated with the progression.28 This further underlines the importance of weight reduction in the treatment of NAFLD.
There were no mortalities in this series and no patients experienced deterioration in liver function. In fact, our data supports our submission that offering minimally invasive weight loss surgery to patients with advanced liver disease is a superior alternative to placement of these individuals on a liver transplantation list. Severe obesity undoubtedly aggravates the risk status of liver transplant candidates, and there are observations that NAFLD recurs following liver transplantation with some patients rapidly developing fibrosis.19 Surgically-induced weight loss may not only decrease subsequent operative risk, but improve liver function and morphology, effectively eliminating the need for transplantation.
One limitation of this study is in the methodology of obtaining intraoperative liver biopsies. These specimens were not retrieved promptly after induction, but rather towards the end of the case, after the patient had been subjected systemically to anesthetic gases and the livers had sustained some degree of local manipulation and pressure effects from mechanical retraction. Theoretically, these factors may have resulted in influx of neutrophils, resulting in a spuriously exaggerated estimation of inflammation in biopsy specimens. However, this was the method followed uniformly in obtaining all intraoperative liver specimens, essentially negating operator bias.
In conclusion, this study presents anthropometric, biochemical, and histologic evidence of significant improvement of NAFLD after profound weight loss induced by minimally-invasive techniques. The reversal of liver disease was globally apparent across all stages and grades (except in two cirrhotic patients) and this effect was achieved with only minimal morbidity and no mortality. There is an intimate relationship between metabolic syndrome and NAFLD, as shown by the coexistence of both disease processes and, conversely, by their simultaneous regression with weight loss. Further study is needed to more clearly define the biologic interactions between the inflammatory components of obesity, metabolic syndrome, and NAFLD. Such research efforts may ultimately provide the ability to halt, if not reverse, the steady rise of fatty liver disease.
Discussions
Dr. Harvey J. Sugerman (Sanibel, Florida): In the early age of bariatric surgery for severe obesity, devastating liver disease developed as a complication of the jejunoileal bypass procedure and it was thought that weight reduction surgery per se could be responsible. However, the liver injury in those patients was probably secondary to the absorption of bacteria and endotoxin from the overgrowth of bacteria in the bypassed intestine, rather than to the malnutrition that occurred in many patients with this radical malabsorptive operation. The JIB is a clinical example of bacterial translocation as antibodies to endotoxin were found in the joint fluid of those patients who developed migratory polyarthritis. Nonalcoholic fatty liver disease, and its more severe form, NASH, are prevalent complications of severe obesity and it is thought that this will be a greater cause of liver cirrhosis in 20–30 years in types A, B, and C hepatitis combined unless we stop this obesity epidemic.
This study from the University of Pittsburgh is the fifth report documenting a marked decrease in hepatic fat infiltration and significant decrease in bridging fibrosis following gastroplasty, gastric bypass, or biliopancreatic diversion for severe obesity. And this improvement was associated with a marked improvement in the metabolic syndrome (type 2 diabetes, hypertension, dyslipidemia) which is thought to be the cause of nonalcoholic fatty liver disease and NASH in severely obese patients. To be able to prevent or reverse cirrhosis, this is really something, and it confirms Dr. Kral and his colleagues’ report in Surgery last year. My questions for the author are:
You included 3 types of bariatric surgery: Lap Band, sleeve gastrectomy, and gastric bypass. Presumably, bypass of the foregut produces a greater effect on insulin sensitivity and diabetes control, as well as greater weight loss than the other 2 procedures. Did you find this to be correct and was there a greater improvement in NAFLD or NASH with the gastric bypass than with the other operations, separately or combined? You had 29 gastroplasty type procedures as compared to 41 gastric bypass patients, and I wondered if there was a significant difference in weight loss and resolution of NAFLD between these 2 groups. Or are your numbers in these 3 subgroups too few to be able to reach a statistically significant conclusion? Were there differences in response rate between men and women?
Hepatic injury has been postulated to be secondary to toxic cytokines, free fatty acids, leptin, as well as other substances. Studies have shown a decrease in inflammatory mediators following surgically induced weight loss. As with other comorbidities of severe obesity, such as pseudotumor cerebri, surgical treatment might help unravel the pathophysiology of this disease to determine which of these potentially injurious mediators may be causing it, and that could lead to a better nonsurgical treatment. Our hepatologists, by the way, recommend gastric bypass in patients with cirrhosis providing they don't have portal hypertension because it improves the safety of subsequent liver transplantation should it be needed.
This report also represents another study documenting the enormous improvement in the comorbidity of severe obesity following weight reduction surgery: diabetes, hypertension, dyslipidemia, polycystic ovary syndrome, sleep apnea, obesity hypoventilation, GERD, asthma, urinary incontinence, venous stasis disease, pseudotumor cerebri, and a markedly impaired quality of life.
Bariatric surgery is clearly the most powerful, positive intervention in recent medical history. The surgery should be embraced by employers and health insurers rather than throwing up roadblocks to access.
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): In terms of your first question regarding stratification of improvement of fatty liver disease according to the type of operation they received, we did not look into that specifically. But we did determine that increased weight loss is a predictor of improvement; therefore, we can confidently say that those operations that are more effective at producing high degrees of weight loss would in turn be more effective in producing improvement in fatty liver disease.
Your second question was about gender. We did determine that the female gender was a predictor of improvement.
As for amelioration or improvement in the degree of inflammation that occurs with weight loss, there are studies that show that there is a reduction in the amount of cytokine activity as patients lose weight, and TLF alpha has been looked at in great detail, as has tumor necrosis factor, as have interleukins. Free fatty acids we know are injurious to the liver in high amounts and they seem to disrupt the beta oxidation mechanism and lead to mitochondrial dysfunction, and we believe that is one of the mechanisms that eventually lead to fatty liver disease and NASH. I am sure that reduced inflammation has a key role to play as patients improve.
Dr. Bruce M. Wolfe (Carmichael, California): The importance of this study is underscored by the ongoing concern that bariatric surgery may injure the liver. Dr. Sugerman referred to the past history of intestinal bypass and its hepatic complications. Dr. Kral in fact reported a variable response to hepatic disease following the currently used malabsorption procedure. The UC-Davis Liver Transplant Program in recent years has seen 2 patients referred for liver transplant who had undergone gastric bypass, and the question arose should the gastric bypass be reversed before transplantation is considered? My questions are:
First, this study involves just 2% of the total population of patients that underwent bariatric procedures at your institution. Could you say more about the criteria for selection for participation in the study and whether this might have impacted the outcomes?
A related question: These biopsies were done beyond the time when rapid weight loss occurs (mean 14 months). Did you see hepatic function deteriorate in any patients during the phase of rapid weight loss when protein deficiency may occur?
Finally, are you aware of any cases referred to the Pittsburgh Transplant Service for evaluation that had undergone any of the procedures you studied in this report?
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): Your first question was regarding the patient selection, regarding the small proportion of patients who were included in the study in comparison to our total population. And that is because the patients who advanced to have second liver biopsies were those that agreed to have a second liver biopsy. That is a problem, a limiting factor in any of these studies, having to perform invasive techniques on patients. We referred patients to the hepatologists who had advanced liver disease according to their histology, and that is how they got initially selected.
As for the rapid weight loss period, yes, we do notice from time to time that patients’ liver functions do rise during the period of rapid weight loss. As I mentioned, these biopsies were taken in the majority of cases beyond their rapid weight loss period, so actually there may be a transient elevation of liver function and inflammation during the period of rapid weight loss. But we believe this is transient and it subsides and then repair and improvement does occur.
As for the reversal or taking down of a bypass in preparation for transplantation, we would actually recommend the opposite, because rendering the patient susceptible to repeat weight gain and obesity and all its comorbidities would just complicate not only the patient's transplantation operation but the future benefits from such an operation. We have not had patients referred to us for that purpose. In fact, our hepatologists and other physicians in other disciplines refer to us patients who are at the advanced stage of organ failure for surgical weight loss in preparation for transplantation.
Dr. Nancy L. Ascher (San Francisco, California): In the old literature between 5–10% of hepatoma procedures were aborted in patients who were found to have cirrhosis and evidence of portal hypertension. What is the current experience of cirrhosis associated with NASH- nonalcoholic steatohepatitis and portal hypertension?
The second question had to do with a study of the patients who did not reverse their liver pathology. NASH is recurrent after liver transplant as well, even in patients who don't gain weight. So there is clearly another process going on with these patients. Have you studied these patients, who clearly along with recurrent NASH following transplant might use information in delineating pathophysiology?
The third question has to do with alcohol. With the 2-hit hypothesis, it is clear that you don't have to drink more than 30 grams a day to have an effect on the liver. So is your advice to these patients? Are they advised to totally abstain from alcohol following bariatric surgery? Is there any association between social drinking and recurrent NASH?
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): As for the practice of aborting operations when discovering cirrhosis, we do not stop the operation. We continue the operation. And in fact we had very good results with these patients. We published that work last year, in which we found liver improvement in the majority of patients. The only times when we would probably not proceed is in the face of portal hypertension.
Your second question about the pathophysiology and the recurrence of a NASH in patients who undergo liver transplantation for presumed– cirrhosis, that is true. We believe that if the stimulatory mechanisms are still present, if the patients are still overweight, if the patients still have components of the metabolic syndrome, their new livers will experience these insults and patients will have a recurrence of their primary disease.
As for alcohol, that is a very difficult question. There is no real confirmatory test of whether patients take alcohol or not. We strongly advise all our patients who undergo gastric bypass to not drink alcohol, and if so only in minute amounts.
Dr. Michael G. Sarr (Rochester, Minnesota): Two short questions. First, you only had 2 patients with established cirrhosis at the time of gastric bypass, limiting your ability to determine if bridging fibrosis is reversible. Kral's evaluation of the data from the LaValle group published last year in surgery showed that in some patients with established cirrhosis at the time of bariatric surgery, later liver biopsy showed marked improvement of the histology with actual reversal of the cirrhosis in a few patients. Do you think that reversal of established cirrhosis or at least bridging necrosis is possible? Second, are there data to support the statement that the patients with morbid obesity do worse after a liver transplantation than people that do not have morbid obesity? This represents a sizable subpopulation of the transplant group, for instance, at our institution awaiting liver transplantation.
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): Regarding reversal of cirrhosis, I do believe that occurs. I am not exactly sure we understand the mechanism of reversal of fibrosis in these patients with advanced disease but John Kral did a great job of demonstrating that phenomenon.
The second question about morbid obesity in liver transplant patients, there is no question that these patients at are extremely high risk and it is not surprising that they do much poorer and have poorer outcomes than lean individuals who receive liver transplants. We strongly recommend, and in fact we have had very good interactions was our hepatologists, for these patients to a undergo surgical weight loss operation prior to their transplantation. It definitely improves their outcome.
Dr. Carlos A. Pellegrini (Seattle, Washington): Do you have any evidence or have you seen any patients in whom any of the components of metabolic syndrome recur with time? Is it weight-gain related? In other words, how good is the operation and for how long do its effects last?
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): The operation has been shown to be very durable. The gastric bypass, for example, has been shown up to 14 years to produce and sustain an excessive weight loss percent of about 50–60%.
Dr. Carlos A. Pellegrini (Seattle, Washington): I am talking about the metabolic syndrome components.
Dr. Samer G. Mattar (Pittsburgh, Pennsylvania): As long as the patients maintain their weight and not undergo regain, the benefits of the operation in terms of the reduction of the components from metabolic syndrome is also sustained.
Footnotes
Supported in part by the University of Pittsburgh Obesity and Nutrition Research Center (P30 DK046204 to E.B.M.).
Reprints: Philip R. Schauer, MD, Cleveland Clinic Foundation, 9550 Euclid Avenue, Desk A80, Cleveland, OH 94195. E-mail: schauep@ccf.org.
REFERENCES
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. [DOI] [PubMed] [Google Scholar]
- 2.Hoppin AG. Obesity and the liver: developmental perspectives. Semin Liver Dis. 2004;24:381–387. [DOI] [PubMed] [Google Scholar]
- 3.Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222–226. [DOI] [PubMed] [Google Scholar]
- 4.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn GL, Mun EC. Effects of weight loss surgeries on liver disease. Sem Liver Dis. 2004;24:371–379. [DOI] [PubMed] [Google Scholar]
- 6.Silverman EM, Sapala JA, Allepman HD. Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Pathol. 1995;104:23–31. [DOI] [PubMed] [Google Scholar]
- 7.Frantzides CT, Carlson MA, Moore RE, et al. Effect of body mass index on non-alcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J gastrointest surg. 2004;8:849–855. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. [DOI] [PubMed] [Google Scholar]
- 9.Kral JG, Thung SW, Biron S, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48–58. [DOI] [PubMed] [Google Scholar]
- 10.Pories WJ, MacDonald KG Jr., Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55:582S–585S. [DOI] [PubMed] [Google Scholar]
- 11.Diagnosis and Classification of Diabetes Mellitus. Position Statement. Diabetes Care. 2005;28:S37–S42. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 13.Schauer PR, Ikramuddin S, Hamad G, et al. Laparoscopic gastric bypass surgery: current technique. J Laparoendosc Adv Surg Tech A. Aug 2003;13:229–239. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien PE, Brown WA, Smith A, et al. Prospective study of a laparoscopically placed, adjustable gastric band in the treatment of morbid obesity. Br J Surg. 1999;86:113–118. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 16.Scheuer PJ, Lefkowich JH. Disturbances of copper and iron metabolism. In: Liver Biopsy Interpretation. 6th ed. Philadelphia: WB Saunders; 2000. [Google Scholar]
- 17.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer PR. Open and laparoscopic surgical modalities for the management of obesity. J Gastrointest Surg. 2003;7:468–475. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury J, Sanyal AJ. Clinical aspects of fatty liver disease. Sem Liver Dis. 2004;24:349–362. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2 E1 activity in non-diabetic patients with non-alcoholic steatohepatitis. Hepatology. 2003;37:544–550. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi NM, Ahmed MM, Polotsky VY, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir physiol Neurobiol. 2003;136:167–178. [DOI] [PubMed] [Google Scholar]
- 22.Haynes P, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis. 2004;8:535–547. [DOI] [PubMed] [Google Scholar]
- 23.Harrison SA, Fincke C, Helinski D, et al. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20:623–628. [DOI] [PubMed] [Google Scholar]
- 24.Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcoholic-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. [DOI] [PubMed] [Google Scholar]
- 25.Lindor KD, Kowdley KV, Heathcote JE, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. [DOI] [PubMed] [Google Scholar]
- 26.Buchwald H, Lober PH, Varco RL. Liver biopsy findings in seventy-seven consecutive patients undergoing jejunoileal bypass for morbid obesity. Am J Surg. 1974;127:48–52. [DOI] [PubMed] [Google Scholar]
- 27.Dallal RM, Mattar SG, Lord JL, et al. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg. 2004;14:47–53. [DOI] [PubMed] [Google Scholar]
- 28.Fassio E, Alvarez E, Dominguez N, et al. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. [DOI] [PubMed] [Google Scholar]