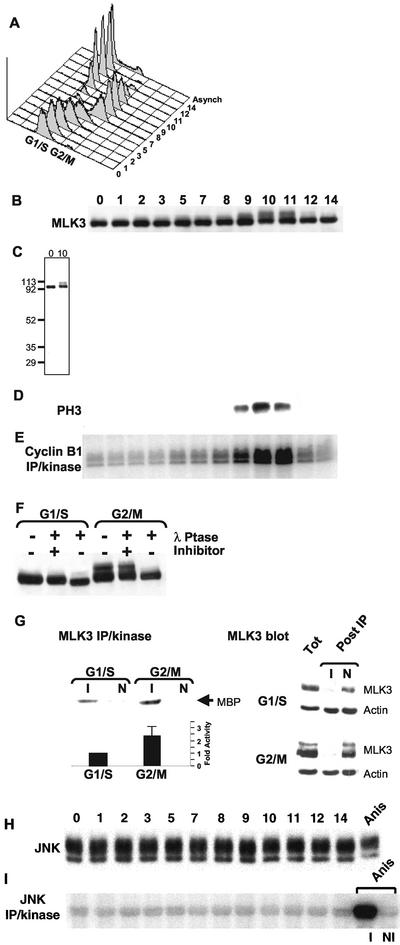
Figure 2.
Cell cycle stimulation of MLK3's phosphorylation state and kinase activity in the absence of JNK activation. HeLa cells were synchronized at G1/S by a double-thymidine block (0) and then released into media lacking thymidine. Samples were collected at subsequent times for 14 h and analyzed as described. (A) Flow cytometric quantification of DNA content per cell (x-axis, propidium iodide stain) vs. cell number (y-axis). Distribution of DNA content of cells from an asynchronous culture is also shown (Asynch). Twenty micrograms of each sample was separated by SDS-PAGE and subjected to immunoblot analysis using anti-MLK3 (B) or antiphosphorylated histone H3 (D). (C) Full-length immunoblot of MLK3 from 0- and 10-h samples. Molecular weight markers are indicated on the left. (E) Anticyclin B1 immune precipitation/kinase assay analysis (100 μg/sample) using histone H1 as substrate. (F) Immunoblot of MLK3 in G1/S (0 h) and G2/M (10 h) samples that were incubated with λ-phosphatase in the presence or absence of the phosphatase inhibitor, EDTA, or were left untreated. (G, upper left panel) MLK3 kinase activity toward myelin basic protein (MBP) was determined for G1/S (0 h) and G2/M (9 h) cell extracts after immunoprecipitation with anti-MLK3 (I) or with nonimmune (N) antibodies. (G, lower left panel) Average values of MLK3 immune precipitation/kinase assays from six different synchronous cultures collected at G1/S (0 h) and G2/M (9, 10, 11 or 12 h, depending on culture). Shown is the average fold stimulation of activity from G2/M samples (2.4 times) relative to G1/S (set at 1) ± the SD (0.67). (G, right panels) Immunoblots of MLK3 and actin in G1/S and G2/M samples before (Tot) and after (Post IP) immunoprecipitation with anti-MLK3 (I) or with nonimmune (N) antibodies. (H) Immunoblot of samples from (A) reacted with anti-JNK antibodies. Twenty micrograms of a sample collected from an asynchronous HeLa culture treated with anisomycin for 20 min was also included (Anis). (I) Anti-JNK immune precipitation/kinase assay analysis (100 μg/sample) of the samples in (H) using GST-N-Jun as substrate. As part of the JNK assays, the anisomycin-treated sample also was subjected to immune precipitation with the anti-JNK antibody (I) or with a nonimmune control antibody (NI) followed by incubation in kinase assay buffer. Products of the kinase reactions (E and H) were separated by SDS-PAGE and substrate incorporation of 32P was detected by phosphorimager exposure.