Figure 1.
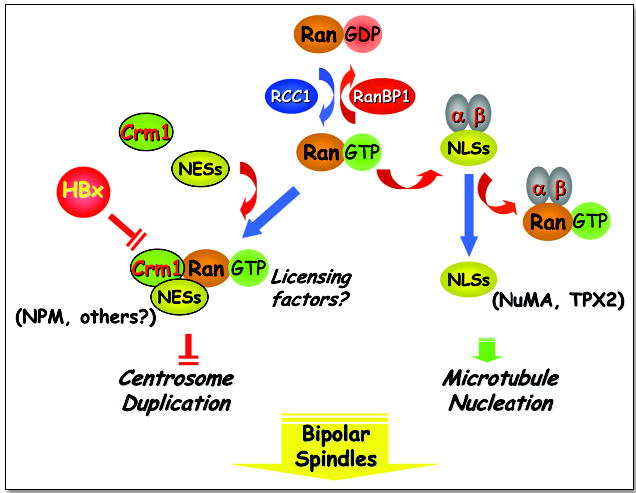
The Ran/Crm1 network: Nucleocytoplasmic transport and mitotic spindle assembly. The small GTPase, Ran, shuttles between an inactive GDP and an active GTP-bound state through interaction with RanBP1 and RCC1 respectively. In its GTP-bound state, Ran can interact with importin receptors (a and b) to promote the cytoplasmic to nuclear transport of proteins containing nuclear localization signals (NLS). The transport of certain NLS-containing proteins such as NuMA and TPX2 can promote microtubule nucleation. Ran-GTP can also interact with the nuclear export receptor, Crm1 that binds to proteins containing nuclear export signals (NES). The hepatitis B viral oncoprotein HBx, interacts with and inactivates Crm1 through its NES, leading to centrosome over-duplication and multipolar spindles. Other NES-containing substrates that bind Crm1, such as nucleophosmin (NPM), may have tumor suppressive effects and function as licensing factors to regulate centrosome duplication during the cell cycle.
