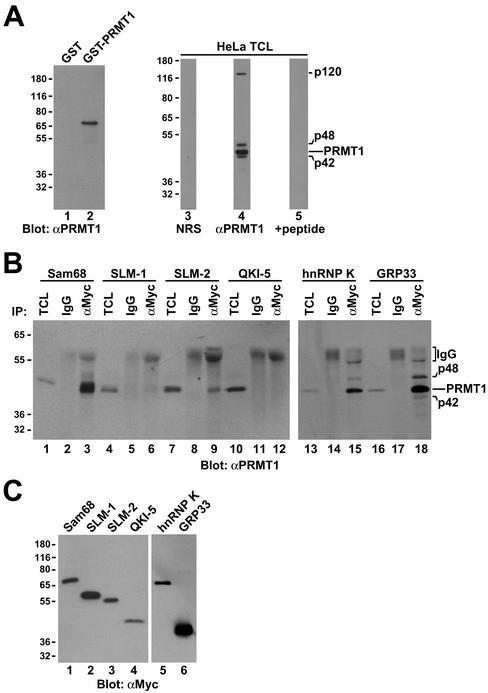
Figure 2.
Association of Sam68 and other KH domain RNA binding proteins with PRMT1. (A) Purified recombinant GST or GST-PRMT1 were separated by SDS-PAGE and immunoblotted with anti-PRMT1 antibodies (lanes 1 and 2). The band at ∼70 kDa represents GST-PRMT1. HeLa cell extracts were separated by SDS-PAGE and immunoblotted with NRS, anti-PRMT1 antibodies, and anti-PRMT1 immunoabsorbed with the antigenic peptide. The migration of the four immunogenic proteins (p120, p48, p45, and p42) recognized by anti-PRMT1 antibodies is indicated on the right (lanes 3–5). The predominant band of ∼45 kDa most likely represents PRMT1 as predicted. The migration of the molecular mass markers in kilodaltons is shown on the left. (B) Myc-tagged Sam68, SLM-1, SLM-2, GRP33, hnRNP K, and QKI-5 were expressed in HeLa and immunoprecipitated with control IgG or anti-myc antibodies. Aliquots of TCLs (10% input) and the immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted with anti-PRMT1 antibodies. The migration of the PRMT1 immunogenic proteins and the heavy chain of IgG are indicated. Longer exposure of lanes 1–12 also reveals p42 and p48 (our unpublished data). (C) Equivalent expression of myc-epitope–tagged KH domain RNA proteins was confirmed by immunoblotting an aliquot of TCLs.