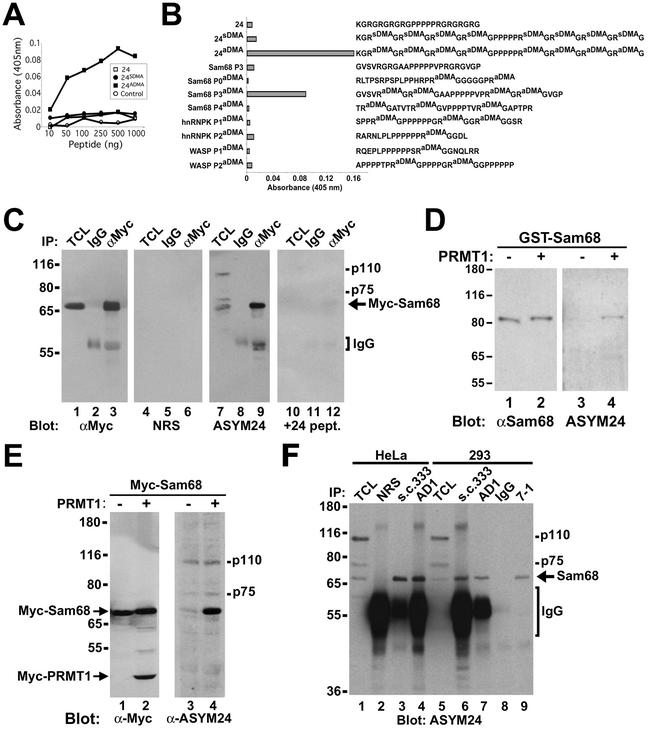
Figure 3.
Sam68 is asymmetrically dimethylated in vivo as detected by using novel anti-aDMA-specific antibodies. (A) Generation of an anti-aDMA antibody. Peptide (24aDMA; see B for sequence) was used as an antigenic peptide to inject rabbits. The specificity of the purified polyclonal antibody (ASYM24) was examined by ELISA with a control unrelated peptide (AD1 peptide), unmethylated “backbone” peptide 24, or an identical peptide containing symmetrical dimethylated arginines (24sDMA). The quantity of peptide bound to the plate was plotted against the immunoreactivity of the ASYM24 antibody. Each point represents the average of three separate experiments. (B) Specificity of the antibody was examined by using a variety of unrelated aDMA containing peptides with neighboring proline-rich sequences. One microgram of the indicated peptides was coated per ELISA well. WASP P1 and P2 denote the Wiscott-Aldrich Syndrome protein proline motifs 1 and 2 containing aDMA. hnRNPK P1 and P2 denote hnRNPK proline motifs 1 and 2 containing aDMA. Sam68 P0, P3, and P4 denote the Src substrate activated in mitosis of 68-kDa proline motifs 0, 3, and 4 containing aDMA. The unmethylated P3 peptide was also used as control (Sam68 P3). Each bar denotes greater than n > 8 from three separate experiments. (C) Myc-tagged Sam68 was transfected in HeLa cells and immunoprecipitated with control (IgG) or anti-Myc antibodies. Aliquots of TCLs (10% input) and the bound proteins were separated by SDS-PAGE and visualized by immunoblotting with anti-myc, NRS, ASYM24, and ASYM24 antibodies absorbed with the antigenic peptide (+24 pept.). The migration of Sam68, the heavy chain of IgG, and endogenous proteins (p110 and p75) is indicated. (D) GST-Sam68 fusion protein was either left untreated (−) or incubated with GST-PRMT1 under in vitro methylation conditions in the presence of “cold” S-adenosyl-l-methionine. An aliquot of each reaction was resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-Sam68 AD1 antibodies (lanes 1 and 2) and ASYM24 (lanes 3 and 4). The molecular mass markers are shown on the left in kilodaltons. (E) HeLa cells were cotransfected with expression vectors encoding myc-Sam68 and myc-PRMT1. The cells were lysed in sample buffer and the proteins separated by SDS-PAGE and immunoblotted with either anti-myc or ASYM24 antibodies. The migration of myc-PRMT1, myc-Sam68, p110, and p75 is indicated. (F) HeLa cells or HEK293 (293) cells were lysed and immunoprecipitated with control antibodies (NRS and mouse IgG) and anti-Sam68 antibodies (pAb s.c.333, pAb AD1, mAb 7-1). The proteins were separated by SDS-PAGE and immunoblotted with ASYM24. The migration of Sam68, the heavy chain of IgG, p110, and p75 is shown on the right. The molecular mass markers are shown on the left.