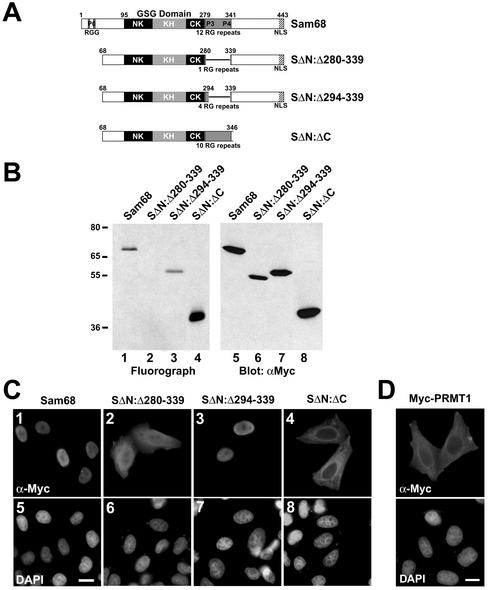
Figure 4.
Sam68 RG-rich sequences are methylated in vivo and regulate protein localization. (A) Schematic diagram representing the various Sam68 proteins is shown. The position of the KH domain and the neighboring N-terminal of KH (NK) and C-terminal of KH (CK) are indicated. The GSG domain encompasses the NK, KH, and CK domains. The gray boxes represent regions where RG repeats are present in Sam68. The C-terminal nuclear localization signal (NLS) of Sam68 is represented by a stippled box. (B) In vivo methylation labeling was performed 24 h after DNA transfection of the expression vectors encoding the proteins depicted in A. The cell lysates were immunoprecipitated with anti-myc antibodies and the 3H-labeled proteins were separated by SDS-PAGE and visualized by fluorography (lanes 1–4; exp. 12 h) and by immunoblotting by using anti-myc antibodies (lanes 5–8). (C) HeLa cells plated on glass coverslips were transfected with myc-epitope–tagged Sam68 (1 and 5), the Sam68 deletion protein that is no longer methylated (SΔN:Δ280–339; 2 and 6), a slightly longer deleted mutant that is methylated (SΔN:Δ294–339; 3 and 7), and a Sam68 missing the C terminus, including the NLS (SΔN:ΔC; 4 and 8). Twenty-four hours after transfection, the cells were fixed and immunostained by using anti-myc antibodies followed by a secondary antibody conjugated to Alexa 546 (red; 1–4). Nuclei were visualized using the DNA-specific stain 4,6-diamidino-2-phenylindole (blue; 5–8).(D) HeLa cells plated on glass coverslips were transfected with myc-epitope–tagged PRMT1 followed by indirect immunofluorescence by using anti-myc epitope-tagged antibodies followed by a secondary antibody conjugated to Alexa 546 (red). Nuclei were visualized using the DNA specific stain 4,6-diamidino-2-phenylindole.