Figure 9.
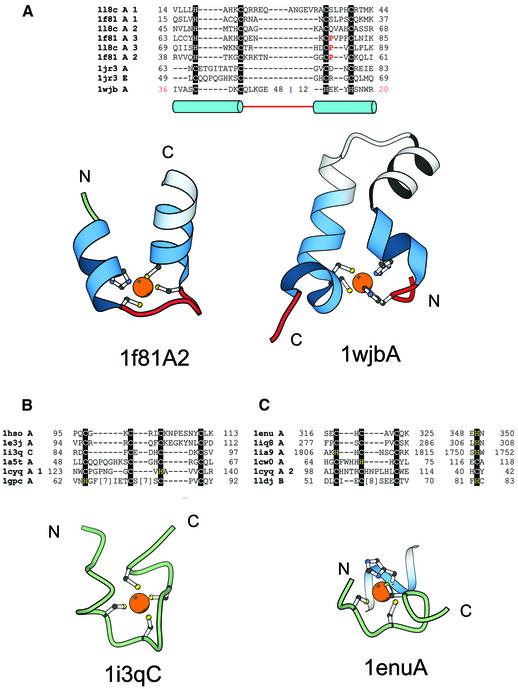
TAZ2 domain-like zinc finger fold group and short zinc-binding loops. (A) Structure-based sequence alignment of members of the TAZ2 domain-like zinc finger fold group. This fold group includes three families, TAZ2 domain family that consists of the structures of the three zinc-binding sites of CBP (1f81A, 1l8cA), the N-terminal zinc-binding domain of HIV-1 integrase (1wjbA) and the zinc-binding domain from DNA polymerase III γ subunit (1jr3A, 1jr3E). Structural diagrams of the second zinc-binding site of the CBP (1f81A2, chain A, residues 38–60) and the N-terminal zinc-binding domain of HIV-1 integrase (1wjbA, chain A, residues 36–48 and 12–20) are shown. Coloring scheme follows that in Figure 1. (B) Structural alignment of short zinc-binding loops present in large protein chains. The proteins in the alignment include loops from the human α alcohol dehydrogenase (1hsoA), sorbitol dehydrogenase (1e3jA), the 45 kDa polypeptide Rpb3 of the DNA-directed RNA polymerase II (1i3qC), the δ′ subunit of the clamp-loader complex of DNA polymerase III (1a5tA), intron encoded homing endonuclease I-PpoI (1cyqA) and core Gp32 ssDNA-binding protein (1gpcA). A representative figure of the RNA polymerase II protein Rpb3 (1i3qC, chain C, residue 84–97) is shown. (C) In some proteins the fourth ligand comes from a secondary structure far away in sequence from the other three ligands. This subgroup includes members from the tRNA-guanine transglycosylase (1enuA, 1iq8A), protein kinase domain of a Trp Ca-channel (1ia9A), intron encoded homing endonuclease I-PpoI (1cyqA), the Vsr endonuclease (1cw0A) and the RING finger protein Rbx1 (1ldjB). A representative figure of the zinc-binding region from tRNA-guanine transglycosylase (1enu, chain A, residue 316–325; 348–350) is shown. Three of the ligands of the zinc-binding site are from a loop and the fourth is from an α-helix.