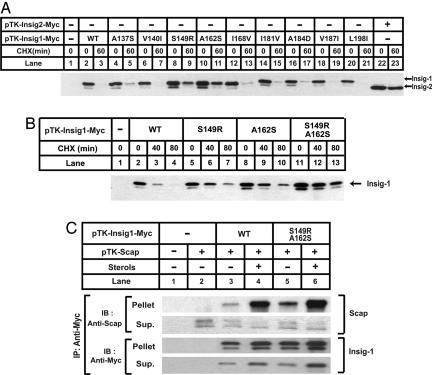
Fig. 3.
Insig-1 is stabilized by mutations at S149 and A162. (A and B) SRD-13A cells were set up at 3.2 × 105 per 60-mm dish on day 0. On day 2, cells in each dish were transfected with 0.5 μg of indicated plasmids. Total plasmid concentration was adjusted to 2 μg per dish by using empty vector pcDNA3.1. On day 3, cells were treated with 50 μM cycloheximide (CHX) for the indicated time. Cells were then harvested, and cell lysate was subjected to SDS/PAGE and immunoblot analysis with anti-Myc IgG-9E10. Filters were exposed for 1 min. (C) SRD-13A cells were set up at 3.2 × 105 per 60-mm dish on day 0. On day 2, cells in each dish were transfected with 1.5 μg of pTK-Scap, and 0.5 μg of wild-type or mutant pTK-Insig1-Myc, as indicated. On day 3, cells were switched to medium B containing 1% hydroxypropyl-β-cyclodextrin. After incubation for 1 h at 37°C, cells were washed twice with PBS, switched to medium B in the absence or presence of sterols (10 μg/ml cholesterol plus 1 μg/ml 25-hydroxycholesterol), and incubated for an additional 5 h at 37°C. Cells were then harvested, and cell lysate was immunoprecipitated (IP) with anti-Myc IgG-9E10 to precipitate transfected Insigs. The supernatant (Sup.) and pellet fractions of the immunoprecipitation derived from 0.1 and 0.5 dish of cells, respectively, were subjected to SDS/PAGE and immunoblot analysis with polyclonal anti-Myc and anti-Scap (R139). Filters were exposed for 1 min.