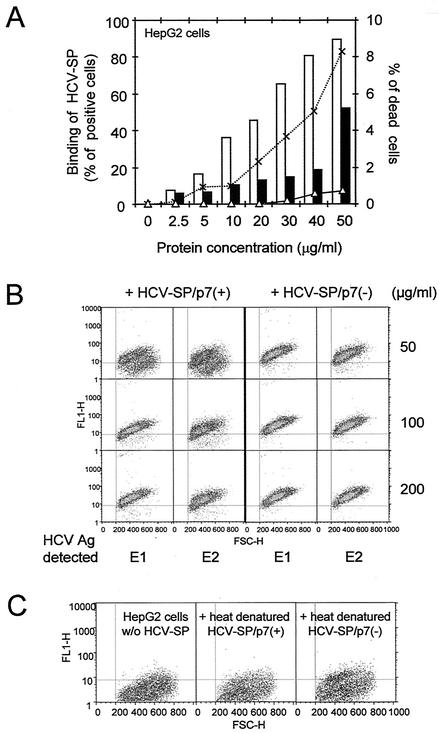
FIG. 3.
Binding of HCV-SP/p7(+) and HCV-SP/p7(−) to HepG2 cells. (A) Binding of light and heavy fractions of HCV-SP to cells. HepG2 cells were incubated with HCV-SP derived from HCV strain 1a as described in Materials and Methods; both light (open bars) and heavy (full bars) fractions were tested. Cell-bound HCV-SP was detected by incubating cells with anti-E2 MAb, followed by FITC-labeled goat anti-mouse IgG and subjecting them to fluorescence-activated cell sorting (i.e., with a FACscan). Nonspecific fluorescence was measured by adding primary and secondary antibodies in the absence of HCV-SP to cells. The cytotoxicity of both light (▵) and heavy (×) fractions was indirectly evaluated by the shift of cell size and the granularity to the bottom left corner of scattered plots. (B) Saturability of the binding of HCV-SP to HepG2 cells. The indicated amounts of proteins from the light fractions of HCV-SP/p7(+) and HCV-SP/p7(−) preparations were incubated with HepG2 cells, and binding was evaluated by using anti-E1 or anti-E2 MAbs with a FACscan as described above. The results are presented on scattered plots: cell granularity is plotted on x axis (FSC-H), whereas the fluorescence intensity is plotted on the y axis (FL1-H); the MFI is calculated for each plot, and the percentages of positive cells were determined according to the threshold values (vertical and horizontal lines) established after the control (in the absence of primary antibody). (C) Inhibition of the binding of HCV-SP to HepG2 cells by heat denaturation. Proteins from the light fractions of HCV-SP/p7(+) and HCV-SP/p7(−) preparations were heated at 90°C for 10 min in the binding buffer; 50 μg/ml was then incubated with HepG2 cells, and the extent of binding was measured as in panel B.